Article contents
Chemographic exploration of the hyalotekite structure-type
Published online by Cambridge University Press: 28 February 2018
Abstract
The hyalotekite group has been approved by the Commission on New Minerals, Nomenclature and Classification of the International Mineralogical Association (memorandum 57–SM/16). The general formula of the minerals of the hyalotekite group may be written as: A2B2M2[Si8T4O28]W where A = Ba2+, Pb2+ or K+; B = Ba2+, Pb2+ or K+; M = Ca2+, Y3+ or REE3+; T = Si4+, B3+ or Be2+; and W = F– or □ (where REE = rare-earth elements and □ = vacancy).
Four minerals are currently known in this group: hyalotekite, Ba4Ca2[Si8B2(SiB)O28]F, triclinic, I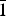 $\bar 1$; khvorovite, Pb2+4Ca2[Si8B2(SiB)O28]F, triclinic I
$\bar 1$; khvorovite, Pb2+4Ca2[Si8B2(SiB)O28]F, triclinic I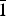 $\bar 1$; kapitsaite-(Y), Ba4(YCa)[Si8B2B2O28]F, triclinic, I
$\bar 1$; kapitsaite-(Y), Ba4(YCa)[Si8B2B2O28]F, triclinic, I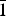 $\bar 1$; and itsiite Ba4Ca2[Si8B4O28]□, tetragonal, I
$\bar 1$; and itsiite Ba4Ca2[Si8B4O28]□, tetragonal, I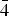 $\bar 4$2m.
$\bar 4$2m.
We explore the possible end-member compositions within this group by conflating the properties of an end-member with the stoichiometry imposed by the bond topology of the hyalotekite structure-type and the crystal-chemical properties of its known constituents. There are two high-coordination sites in the hyalotekite structure, A and B, and occupancy of each of these sites can be determined only by crystal-structure refinement. If these two sites are considered together, there are 19 end-member compositions of the triclinic structure and six end-member compositions of the tetragonal structure involving A and B = Ba2+, Pb2+, K+; M = Ca2+, Y3+, REE3+; and T = Si4+, B3+, Be2+. There is the possibility for many other hyalotekite-group minerals, and two potential new minerals have been identified from data in the literature.
Keywords
- Type
- Article
- Information
- Copyright
- Copyright © Mineralogical Society of Great Britain and Ireland 2018
Footnotes
Associate Editor: G. Diego Gatta
References
- 3
- Cited by




