Article contents
Biphosphammite: a second occurrence
Published online by Cambridge University Press: 05 July 2018
Summary
Biphosphammite, NH4H2PO4, recorded once as secondary powder in 1870, occurs with bat guano in Murra-el-elevyn cave, 31° 20′ S., 126° 0′ E., Western Australia.
The mineral occurs as tapering tetragonal prisms with pyramidal terminations, which are colourless to light buff, very soft, water soluble, contain fine syngenite inclusions, and have D 2·04, ω 1·525, ε 1·480 Space group. 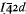 , a 7·4935 Å, c 7·340 Å, six strongest powder X-ray lines 3·75 Å (10) 200; 5·24 Å (9) 101; 3·02 Å (9) 112; 1·993 Å (8) 312,213; 2·65 Å (7) 220; 2·368 Å (7) 310,301. Partial analysis of purest available material gave: P2O5 51·1, K2O 14·2, (NH4)2O 12·3, SO2 5·59, Na2O 0·16, water-insolubles 0·81 per cent, remainder mainly CaO and H2O. Calculated mineral content is 88 % biphosphammite (62 % NH4H2PO4, 38% isomorphous KH2PO4), 11·5% syngenite, insolubles 0·81 % calc. total 100·3 per cent.
, a 7·4935 Å, c 7·340 Å, six strongest powder X-ray lines 3·75 Å (10) 200; 5·24 Å (9) 101; 3·02 Å (9) 112; 1·993 Å (8) 312,213; 2·65 Å (7) 220; 2·368 Å (7) 310,301. Partial analysis of purest available material gave: P2O5 51·1, K2O 14·2, (NH4)2O 12·3, SO2 5·59, Na2O 0·16, water-insolubles 0·81 per cent, remainder mainly CaO and H2O. Calculated mineral content is 88 % biphosphammite (62 % NH4H2PO4, 38% isomorphous KH2PO4), 11·5% syngenite, insolubles 0·81 % calc. total 100·3 per cent.
Material proposed to be neotype is preserved at the Government Chemical Laboratories, Perth, Western Australia.
- Type
- Research Article
- Information
- Copyright
- Copyright © The Mineralogical Society of Great Britain and Ireland 1972
References
- 8
- Cited by




