Article contents
The aqueous chemistry of uranium minerals. Part I. Divalent cation zippeïte
Published online by Cambridge University Press: 05 July 2018
Synopsis
Free energies of formation of divalent metal ion zippeïtes, M2(UO2)6(SO4)3(OH)10 ·nH2O, M = Mg,Co,Ni,Zn have been determined from solution studies and metal speciation calculations, in water. It is found that in the compounds, the number of molecules of water of crystallization is equal to 8. This is at variance with a previous report (Frondel et al., 1976), but it has been found that some at least of the water content of zippeite is either nonessential or very loosely bound in the structure. Based on the octahydrate formulation, 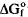 values are −3506, −12695, −12683 and −12870±4 kJ mol−1 for the Mg,Co,Ni and Zn end-members, respectively. Almost all of the differences in the
values are −3506, −12695, −12683 and −12870±4 kJ mol−1 for the Mg,Co,Ni and Zn end-members, respectively. Almost all of the differences in the 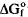 values are accounted for by those values for the metal ions alone with the exception of Znzippeïte where a discrepancy of some 22 kJ mol−1 is found. Even this value is small however, and the chemical studies indicate that extensive mutual solid solution between all end members is to be expected. These findings agree perfectly with observations on the composition of naturally occurring zippeite minerals of this group.
values are accounted for by those values for the metal ions alone with the exception of Znzippeïte where a discrepancy of some 22 kJ mol−1 is found. Even this value is small however, and the chemical studies indicate that extensive mutual solid solution between all end members is to be expected. These findings agree perfectly with observations on the composition of naturally occurring zippeite minerals of this group.
- Type
- Research Article
- Information
- Copyright
- Copyright © The Mineralogical Society of Great Britain and Ireland 1979
References
Reference
References
- 7
- Cited by




