Article contents
Cuyaite, Ca2Mn3+As3+14O24Cl, a new mineral with an arsenite framework from near Cuya, Camarones Valley, Chile.
Published online by Cambridge University Press: 27 April 2020
Abstract
Cuyaite (IMA2019-126), Ca2Mn3+As3+14O24Cl, is a new arsenite mineral from near Cuya in the Camarones Valley, Arica Province, Chile. It is associated with anhydrite, native arsenic, arsenolite, calcite, claudetite, ferrinatrite, gajardoite-3R, leiteite, magnesiocopiapite, phosphosiderite, pyrite, realgar and talmessite and formed from the oxidation of As-bearing primary phases and alteration by saline fluids derived from evaporating meteoric water under hyperarid conditions. Cuyaite occurs as pale brown thin needles (elongated on [010]), typically in divergent sprays and subparallel intergrowths. The streak is white. Crystals are transparent with adamantine lustre; subparallel intergrowths exhibit silky lustre. The mineral has Mohs hardness of 2½, is brittle, exhibits no cleavage and has irregular fracture. The calculated density is 4.140 g cm–3. Cuyaite is optically biaxial (–), with α = 1.87(1), β = 1.956(calc) and γ = 1.98(1), determined in white light; 2Vmeas = 60(1)°; and orientation: X = b and Y ^ a = 53° in obtuse β. Electron microprobe analyses provided the empirical formula Ca2.03Mn3+0.95(As3+13.66Sb3+0.65)Σ14.31O24Cl0.88. The six strongest powder X-ray diffraction lines are [dobs Å(I)(hkl)]: 4.73(45)(111, 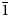 $\bar{1}$12), 3.162(100)(
$\bar{1}$12), 3.162(100)(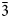 $\bar{3}$14), 3.035(28)(213), 3.004(37)(204), 2.931(90)(
$\bar{3}$14), 3.035(28)(213), 3.004(37)(204), 2.931(90)(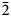 $\bar{2}$15, 312) and 2.779(28)(020). Cuyaite is monoclinic, Pn, a = 14.7231(6), b = 5.58709(19), c = 17.4185(12) Å, β = 112.451(8)°, V = 1324.23(14) Å3 and Z = 2. In the crystal structure of cuyaite (R1 = 0.0369 for 2095 I > 2σI reflections), AsO3 pyramids share O corners to form a ‘loose’ 3D framework; Jahn–Teller distorted Mn3+O6 octahedra and CaO8 polyhedra link by edges and corners to form columns; the columns also link by edge- and corner-sharing to the AsO3 pyramids in the framework; Cl occupies channels along [010] in the framework. The Raman spectrum is consistent with the presence of multiple As3+O3 groups.
$\bar{2}$15, 312) and 2.779(28)(020). Cuyaite is monoclinic, Pn, a = 14.7231(6), b = 5.58709(19), c = 17.4185(12) Å, β = 112.451(8)°, V = 1324.23(14) Å3 and Z = 2. In the crystal structure of cuyaite (R1 = 0.0369 for 2095 I > 2σI reflections), AsO3 pyramids share O corners to form a ‘loose’ 3D framework; Jahn–Teller distorted Mn3+O6 octahedra and CaO8 polyhedra link by edges and corners to form columns; the columns also link by edge- and corner-sharing to the AsO3 pyramids in the framework; Cl occupies channels along [010] in the framework. The Raman spectrum is consistent with the presence of multiple As3+O3 groups.
Keywords
- Type
- Article
- Information
- Copyright
- Copyright © The Mineralogical Society of Great Britain and Ireland 2020
Footnotes
Associate Editor: Irina O. Galuskina
References
- 1
- Cited by




