No CrossRef data available.
Article contents
In-Situ Growth and Polygonization of Epitaxial Passive Oxide Films on Nanoparticles of Iron
Published online by Cambridge University Press: 02 July 2020
Abstract
Over the years, the study of the oxidation of nanoparticles of iron by transmission electron microscopy (TEM), Mossbauer spectroscopy and X-ray diffraction has established that nanoparticles of iron have a core-shell morphology in which the iron core is enclosed by shell of polycrystalline shell of ultrasmall γ-Fe2O3 and Fe3O4 crystallites. Recently, passivated nanoparticles of iron prepared by gas condensation of plasma evaporated vapor in Tianjin University exhibit remarkable resistance to further oxidation and corrosion in air and water. We have showed by TEM that these nanoparticles of iron are protected by a 4 nm epitaxial shell of γ-Fe2O3. The epitaxial orientation relationship, established by convergent beam electron diffraction from a nanoparticle, is as follows:
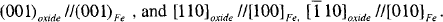
The [001] diffraction pattern of the oxide is rotated by 45° about a cubic axis relative to that of iron.
- Type
- Thin Films & Coatings
- Information
- Copyright
- Copyright © Microscopy Society of America 2001




