No CrossRef data available.
Article contents
Ultrastructural and Molecular Development of the Myotendinous Junction Triggered by Stretching Prior to Resistance Exercise
Published online by Cambridge University Press: 08 March 2022
Abstract
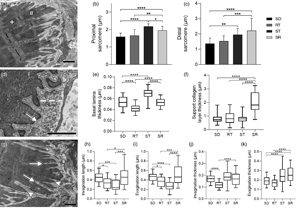
The myotendinous junction (MTJ) is a highly specialized region of the locomotor apparatus. Here, we investigated the ultrastructural and molecular effects in the MTJ region after static stretching prior to the ladder-based resistance training. Thirty-two male, 60-day old Wistar rats were divided into four groups: Sedentary, Resistance Training, Stretching, and Stretching-Resistance Training. The gastrocnemius muscle was processed for transmission electron microscopy techniques and Western blot assay. We observed that the static stretching prior to the ladder-based resistance training increased the MTJ components, the fibroblast growth factor (FGF)-2 and FGF-6 protein expression. Also, we demonstrated the lower transforming growth factor expression and no difference in the lysyl oxidase expression after combined training. The MTJ alterations in response to combined training demonstrate adaptive mechanisms which can be used for the prescription or development of methods to reduce or prevent injuries in humans and promote the myotendinous interface benefit.
- Type
- Biological Applications
- Information
- Copyright
- Copyright © The Author(s), 2022. Published by Cambridge University Press on behalf of the Microscopy Society of America
Footnotes
These authors contributed equally to this study.



