We examine the impact that a small and isolated society had on ridgetop forest environments far from easy access to potable water in recent historical-archaeological times. Before contact in 1956, the Waorani, who at that time probably numbered no more than 500, lived in small family groups on interfluvial ridgetops far from water. This study is based on a systematic examination of these ridgetops and on interviews with older Waorani who were born before contact in the late 1950s.
Landscapes of the Upper Amazon, including the research area—the interfluvial region between the Napo and Pastaza Rivers of Ecuador—have long been seen by outsiders as pristine. The small-scale societies such as the Waorani who live there were dismissed as minor hunter-gatherers who had no long-term impact on their environment (e.g., Blomberg Reference Blomberg and Lyon1957; cf. Up de Graff Reference Up de Graff1921). At the same time, much evidence suggests that people lived in this region for thousands of years. Did they really have no historical impact? The historian Miguel Cabodevilla (Reference Cabodevilla1994:13) asked, “Do the Waorani have prehistory, or are they prehistory themselves?” We propose that the Waorani did have a history, one written in the altered composition of the ridgetop forests where they lived during wartime.
The Napo River basin lies within the loosely defined archaeological area known as the Upper Amazon, the watershed of which extends from the easternmost slopes of the Andes—below the tree line—to the mouth of the Madeira River (Clasby and Nesbitt Reference Clasby and Nesbitt2021; Lathrap Reference Lathrap1970); hence, it covers many distinct landforms with dissimilar elevations, plant physiognomies and species, fauna, rainfall amounts, seasonality, soil conditions, and the like. For the most part, the Napo River basin is heavily covered by mature rainforest, where there are no towns, small farms, or oil extraction roads. These forests are the most biologically diverse of any region in the Upper Amazon, and indeed the Amazon Basin: almost every other individual tree on a typical forest plot is a different or new species (Dangles et al. Reference Dangles, Nowicki and Renato2009; Gentry Reference Gentry1988). As we argue here, some of the mature forest sometimes shows effects of Indigenous management or disturbance in the past, even if that past coincides with conventionally defined historical time periods (cf. McMichael et al. Reference McMichael, Piperno, Bush, Silman, Zimmerman, Raczka and Lobato2012; Piperno et al. Reference Piperno, McMichael and Bush2015).
No archaeological investigations have been carried out at the study sites reported here (Zurita-Benavides Reference Zurita-Benavides2014:29). The only such investigations in Waorani Territory thus far are in its northern part (Netherly Reference Netherly1997), the area south of the Napo River and north of the Nushiño and Curaray River basins. For the most part, the archaeological periodification proposed by Evans and Meggers (Reference Evans and Meggers1968) is little changed (Arroyo-Kalin and Rivas Panduro Reference Arroyo-Kalin and Panduro2019), and few 14C dates for the region are published (Rostain and Saulieu Reference Rostain, de Saulieu and Muriel2013). No archaeological excavations have been conducted inside the Yasuni National Park. Much of the recent archaeological work has been in contract archaeology and remains in reports or is unpublished (Arroyo-Kalin and Rivas Panduro Reference Arroyo-Kalin and Panduro2019). Agriculture is nevertheless ancient in the region because maize is known from 6,000 years ago (Bush et al. Reference Bush, Piperno and A1989) to the west, therefore implying the presence of archaeological sites in the area.
Our focus is on ridgetop forests whose history and origin have yet to be studied at all. As to the archaeological sequence in Waorani Territory, three phases associated with distinctive pottery types are usually recognized: the Yasuni Phase from around 50 BC–AD 500, which is considered part of the Formative period; the Tivacuno Phase from about AD 500–700; and the Napo Phase from about AD 1000–1500. Because no ceramics used in the processing of bitter manioc have been found, some researchers speculate that the Yasuni and Tivacuno Phases were associated with trekking people or incipient horticulturalists cultivating only sweet manioc, sweet potatoes, and perhaps other crops that do not require detoxification technology (Evans and Meggers Reference Evans and Meggers1968:18, 33; Porras Reference Porras1987:245; Rostain and Saulieu Reference Rostain, de Saulieu and Muriel2013:116). The Napo Phase consists of sophisticated anthropomorphic pottery, including elaborate funerary urns (Arroyo-Kalin and Rivas Panduro Reference Arroyo-Kalin and Panduro2019:344); it is associated with the grander Amazon Polychrome Tradition (Arroyo-Kalin and Rivas Panduro Reference Arroyo-Kalin and Panduro2019; Rostain and Saulieu Reference Rostain, de Saulieu and Muriel2013) and perhaps with the cultivation of bitter manioc, based on material culture (Porras Reference Porras1987:264). The Napo Phase of the Amazon Polychrome Tradition seems to have been replaced by or mixed with Omagua pottery seen initially by Orellana in his descent of the Napo and ultimately the Amazon River itself in AD 1541–1542 (Rostain and Salieu Reference Rostain, de Saulieu and Muriel2013). After the European conquest, Omagua people—already intrusive Tupí-Guaranian–speaking people originating from the east—ascended the Tiputini River, among other rivulets close to the Napo itself, and may have driven other groups out of the area (Cabodevilla Reference Cabodevilla2013). In the region of study, the Waorani were evidently preceded by Zaparoan groups who by the early twentieth century were almost completely gone (Cipolletti Reference Cipolletti2002).
As mentioned, agriculture is ancient, because maize is known from a lakebed just to the north of Waorani Territory to have been cultivated 6,000 years ago (Bush et al. Reference Bush, Piperno and A1989). It is likely that domesticated crops native to the Amazon including tree crops—especially the edible variety of the peach palm Bactris gasipaes Mart.—precede that time period by several thousand years and were spread all over the area, as they are today (Clement et al. Reference Clement, de Cristo-Araújo, D'Eeckenbrugge, Pereira and Picanço-Rodrigues2010; Levis et al. Reference Levis, Flores, Moreira, Luize, Alves, Franco-Moraes and Lins2018). Arguably, people have altered lowland forests in the region since the Holocene (McMichael and Bush Reference McMichael and Bush2019), perhaps permanently.
Research since initial peaceful contact shows the existence of several atomistic sociopolitical subgroups or clusters of the Waorani (e.g., Cipolletti Reference Cipolletti2002; Zurita-Benavides Reference Zurita-Benavides2014). The research reported here focuses only on those groups in the Curaray River basins and its affluent, the Nushiño River basin. We present evidence for human forest management on the interfluvial ridgetops in the southern part of Waorani Territory, all of which is south of the Napo River itself. Although there is no Amazon Dark Earth or major earthworks, in contrast to the Lower and Central Amazon (McMichael and Bush Reference McMichael and Bush2019), we argue, using original data, that the tree composition on these ridgetops has been altered by human—specifically Waorani—interaction with their forest dwelling sites: the ridgetop forests are anthropogenic and differ from undisturbed or minimally disturbed forests in the same region.
Cultural Forests
The idea that humans have contributed to the makeup of Amazonian forests seems obvious, especially when considering alpha diversity: species counts that are systematically measured in localized, controlled settings (see Levis et al. Reference Levis, Flores, Moreira, Luize, Alves, Franco-Moraes and Lins2018). Arguably the collapse of any large primate population, such as woolly monkeys or howler monkeys, will alter seed distribution and hence the makeup of the forest (e.g., Redford Reference Redford1992). If this is the case with woolly monkeys, human beings could have had a similar effect on the dispersal of seeds that, in some cases, become viable and mature into trees. In the Lower Amazon, nondomesticated cacao (Theobroma speciosum Mart.), for example, is dispersed both by Indigenous humans (e.g., the Ka’apor people) and capuchin monkeys (Balée Reference Balée1994:149).
The Indigenous Kichwa-speaking peoples of Zaparoan descent who live between the Napo and the Pastaza Rivers take it for granted that their forests were the gardens of the Tayag, their name for the original inhabitants of the area. These inhabitants, not unlike the Dreaming Beings of Aboriginal Australia, were anthropomorphized to be sure, but were likely to have been Homo sapiens capable of art, ceramics, architecture, and—most important for our purpose here—landscape modification (Balée Reference Balée2018; Crumley et al. Reference Crumley, Lennartsson and Westin2018). Romantic Western ideas of virgin forest, untouched by the human hand or, as Thoreau put it in 1854 (Reference Thoreau and Cramer2004), “never profaned by any human neighborhood,” have been difficult to shake even into the twenty-first century—especially for speciose landscapes like those of Amazonia. Regardless of romanticism, a widespread myth of untouched, pristine, virgin forest has been rejected by research demonstrating how the Indigenous populations of North America (Cronon Reference Cronon1983; Pyne Reference Pyne1982) or even the Lower Amazon contributed to the makeup of ancient forests (e.g., Balée Reference Balée1989; Denevan Reference Denevan1992; Heckenberger et al. Reference Heckenberger, Christian Russell, Fausto, Toney, Schmidt, Pereira, Franchetto and Kuikuro2008; Levis et al. Reference Levis, Costa, Bongers, Peña-Claros, Clement, Junqueira and Neves2017, Reference Levis, Flores, Moreira, Luize, Alves, Franco-Moraes and Lins2018; Rostain Reference Rostain2021; Winklerprins and Levis Reference WinklerPrins and Levis2021).
Until now, however, the Upper Amazon has tended to be seen as not having intense human modifications: this view was supported by research into the floodplain forests. Based on a study of two lake districts in Amazonian Peru and Ecuador, paleoecologist Mark Bush and colleagues (Reference Bush, Silman, de Toledo, Listopad, Gosling, Williams, de Oliveira and Krisel2007:216) concluded, “Modern biodiversity and forest composition are not a product of land management, but have persisted despite it” (also see McMichael et al. Reference McMichael, Piperno, Bush, Silman, Zimmerman, Raczka and Lobato2012; Piperno et al. Reference Piperno, McMichael and Bush2015). The hypothesis was that Indigenous impacts in prehistory were negligible over the long term and could not be used to explain current patterns of diversity in Amazonia, though that hypothesis has become more nuanced recently. McMichael and Bush (Reference McMichael and Bush2019) note that much of the western Amazon biological diversity was influenced by human activity in the Holocene. Our argument is that alpha (highly local) diversity in Upper Amazonian forests has at times been affected by anthropogenic means, which can be shown by combining Indigenous knowledge with forest inventories on ridgetops.
Historical-ecological arguments in support of long-term human impacts on Indigenous forests have recently been proposed (e.g., Levis et al. Reference Levis, Costa, Bongers, Peña-Claros, Clement, Junqueira and Neves2017, Reference Levis, Flores, Moreira, Luize, Alves, Franco-Moraes and Lins2018; Stahl Reference Stahl2015). In Amazonia, it is conceivable that the temporary manioc gardens made by bands of hunter-gatherers along the floodplains of fast-flowing rivers like the Napo and its Upper Amazonian tributaries were simply washed away along with the volcanic silt these streams carry down from the Andes—just as the lack of Amazon Dark Earth and of evidence of large settlements in the vicinity of the monumental geoglyphs of Acre, Brazil, could be the result of meandering river flooding by the Purus River and its tributaries (Schaan Reference Schaan2011). Because these rivers change course rapidly, any effect of manioc gardening might have been covered with more volcanic silt. Such a scenario, however, is not the case with the ridgetop forests in the Nushiño and Curaray basins because they never flood, given their elevation above river floodplains.
An Isolated Language and Society
All Waorani speak a complex, difficult-to-analyze language isolate (Aikhenvald Reference Aikhenvald2012:52, Map 1.7B; Peeke Reference Peeke1973): Wao Tededo. It has no living sister languages and pertains to no known family of languages (Wasserstrom Reference Wasserstrom2016; Figure 1). Culturally and historically, little is known of the Waorani before the great Amazonian rubber boom, but it is clear that, at least from the late nineteenth century through the mid-1960s, they sought to avoid contact with the outside world. One Ecuadorian official wrote in 1887 that there were more than 500 caucheros (caucho: Castilla ulei; rubber gatherers) in the Napo River basin alone who had “committed many abuses against Indians” (Stanfield Reference Stanfield1998:58). What contact the Waorani had during and after the rubber boom, with few exceptions, was hostile: they conducted raids on caucheros and other interlopers in their lands to obtain steel tools (Wasserstrom Reference Wasserstrom2016), and tiny Waorani groups raided other such groups (Long Reference Long2019; Robarchek and Robarchek Reference Robarchek and Robarchek1998). Peaceful contact with some subgroups dates from 1958, after six Wao men speared to death five evangelical Christian missionaries who had landed a small plane on a beach in the Curaray River (Long Reference Long2019:14–21). By 1975, most of the Waorani had been resettled to the Summer Institute of Linguistics mission station at Tewæno. After that time hostilities largely ceased. The Waorani families gradually moved from the mission to settle along the rivers where they now live, hunting, fishing, and farming sweet manioc mainly (Long Reference Long2019). At least two small groups of Waorani-language speakers referred to as the Tagædi (“Tagæ's bunch”) and Taadomenani (the “lowlanders or meandering ones”; Long Reference Long2019:342) continue to live in “voluntary isolation” in the area called the zona intangible (intangible zone) inside Ecuador's Yasuni National Park (Cabodevilla Reference Cabodevilla2010; Finer Reference Finer, Vijay, Ponce, Jenkins and Kahn2009; Long Reference Long2019:182, 342).
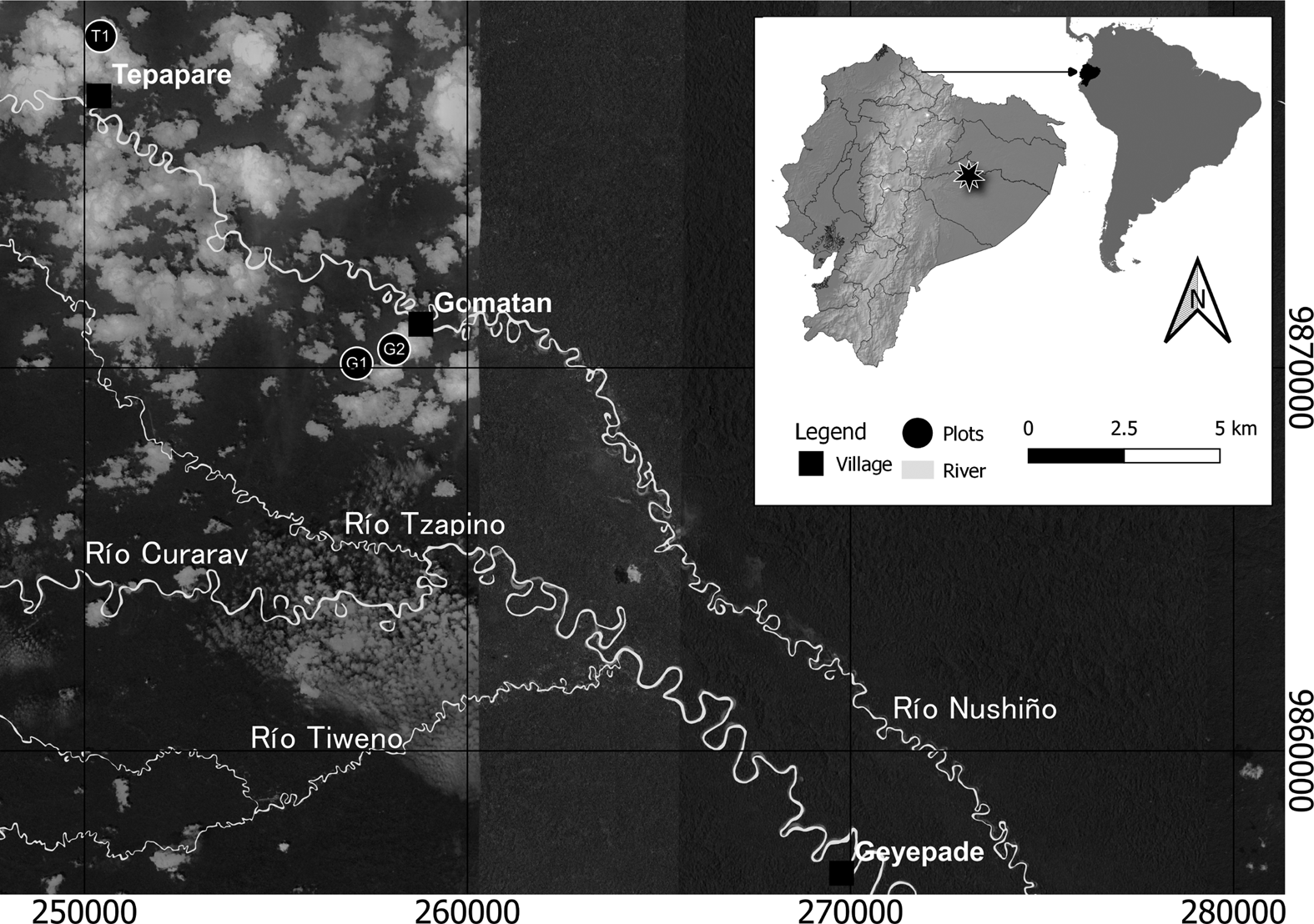
Figure 1. Area of research and inventory sites.
During periods of conflict before contact with missionaries in the late 1950s and 1960s, our Wao collaborators tell us that Waorani ancestors preferred to live secluded on interfluvial ridgetops far from water, where they could avoid detection and from where they could more easily defend their homes. One kin-based political subgroup was called the “Ridge People” (Weipedi; Long Reference Long2019:162, 198), even though all the Waorani were living on ridgetops before contact. The Weipedi seemed invisible to the outside world—including to other Indigenous peoples in the environs—except when they conducted raids on settlements. They were living in a situation like that of a few Brazilian Indigenous groups today in the southwest Amazon (e.g., Moriconi and Londoño Reference Moriconi and Londoño2020), who could also be linguistic and cultural relatives of groups that have a long history of continuous contact with national societies. In the 1940s when the Swedish explorer Rolf Blomberg (Reference Blomberg and Lyon1957) carried out a canoe expedition down the Río Nushiño through the territory of the Guiquitaidi— “Guiquita's bunch” or cluster, a small kin-based, political subgroup of the Waorani, who accounted for about 10% of the entire Waorani population (Long Reference Long2019:15)—he was unable to locate any people. In fact, they were there but were safely hidden in dwellings on ridgetops, including ridgetops where the research sites reported here were situated. The isolated relatives of today—the Tagædi and Taadomenani—have an archetypical ancestral way of life. They are nearly naked trekkers—the men wear only a hip-cord known as a come—who establish small swiddens of manioc and plantain using stone axes and continue to reside on hidden ridgetops: in a real sense, they are still living in wartime mode (Long Reference Long2019:192).
Little is known about Waorani history in the colonial period. The names of most rivers between the Colombian border and the Río Pastaza and in a band running from the Andean foothills to the Peruvian border end in -no/ro, which are allophones for the same suffix used to designate rivers. Wao Tededo—the Waorani language—is the only language known to have this suffix for “river.”Footnote 1 Although the morphologically stand-alone terms for river are “epene” and “ononga,” when a given river is named, it is postposed to the name with the suffix -no/ro. Contemporary Waorani do not recognize the meaning of the first part of river names in the larger area surrounding their territory, such as Araj-uno, Cuyab-eno, Payam-ino, and Vill-ano.
One of the most important species that enabled the Waorani to flourish far from rivers on the ridgetops in times of war was the massive, solitary, slow-growing Oenocarpus bataua Mart. palm (Rival Reference Rival2016:52). Regardless of Waorani prehistory and possible sedentism in this region, one may speculate that, on ridgetops high above the southern tributary waters of the Napo, the Waorani people—in the years before permanent contact by 1960—may have consumed greater quantities of O. bataua mesocarps and discarded the seeds around their homes; this then led to the altering of forest composition. We propose here that the particular way in which this wartime food was prepared, consumed, and discarded on the ridgetops led to a higher density of this palm species, thereby permanently changing the makeup of these ridgetop forests. It seems that in ridgetop forest occupation sites, to make tepe (a drink made from boiled manioc), O. bataua fruits were used in place of peach palm (Bactris gasipaes Mart.) fruits—a domesticated palm (Clement et al. Reference Clement, de Cristo-Araujo, d'Eeckenbrugge, dos Reis, Lehnebach and Picanço-Rodrigues2017) only found in orchards nearest the riverbanks (Cerón and Montalvo Ayala Reference Ceron and Montalvo Ayala1998:180). This explains the abundant presence of the O. bataua palm on the forested ridgetops and its economic importance for those who live near there.
As substantiated in the later discussion, Waorani ancestors’ dispersal of the seeds and protection of seedlings of this economically important palm partly explain why it exhibits dominance among orthogonal plants in the size category of greater than 10 cm in diameter at breast height (1.3 m from the ground) in ridgetop forests. Indigenous groups in the Upper Amazon also consume proteinaceous palm grubs from fallen trees and may also take the heart of palm from juvenile specimens (Balick Reference Balick1986). Laura Rival (Reference Rival2016:52) noted,
The ungurahua [petome or O. bataua] palm provides rich food, building materials, and raw materials for the making of a wide range of artifacts and remedies. Besides being an extremely useful plant resource, the ungurahua palm offers protection. . . . Those who flee from wars and spearing raids would not survive without the ungurahua fruit. It is rich in fats and proteins and ripens throughout the year.
Ethnobotanists who studied a Wao site on the Shiripuno River also observed that O. bataua occurs on bosque de colinas (ridgetop forests) and bosque aluvial (floodplain forests), but not in between floodplains and ridgetops, the zone of the control site we examined (Ceron and Montalvo Ayala Reference Ceron and Montalvo Ayala1998:183).
Historical Background
The interfluvial ridgetops seem to be untouched by human influence because they are the most remote and inaccessible areas of the region. Our hypothesis is that it is just this quality of remoteness from the accessible riverbank fields, which are usually associated with human habitation, that accounts for how and why the ridgetop forests were shaped by people. Before 1956, Waorani tended to avoid contact with both Indigenous and non-Indigenous outsiders because they considered them to be extremely dangerous: they habitually refer to all outsiders as cowode (“barely human cannibals”; Long Reference Long2019:127). Ridgetops would have been strategic locations from which to watch over their territory, identify the presence of any intruders, and determine the ideal time to cross rivers and plant high-energy crops, such as manioc and plantains, in peacetime (Rival Reference Rival2002; Robarchek and Robarchek Reference Robarchek and Robarchek1998:77–78; Wierucka Reference Wierucka2015; Zurita-Benavides et al. Reference Zurita-Benavides, Jarrín and Rios2016). Ridgetops just large enough to support a longhouse containing an uxorilocally extended family (nanicabo) were the preferred domestic space. They also provided refuge for Waorani families in times of war (Wierucka Reference Wierucka2015:71–72).
Elders who were young at the time of first contact describe an interfluvial lifestyle in which small family groups avoided the major rivers except for certain seasons each year. Away from the rivers, they lived on monkeys and birds killed with blowguns, as well as white-lipped peccary and fish from small inland streams. During peaceful times this diet was supplemented with starch from cultigen staples such as plantains, sweet manioc, and peach palms. The latter two plants were often consumed in the liquid form of tepe.
Rival (Reference Rival2002, Reference Rival2016) describes a pattern of circular migration in which small Waorani bands would trek between inland peach palm groves to manage sites where trees had been planted in past generations. During times of war, which occurred frequently between 1910 and 1950, these agricultural foods became less accessible. Even inland peach palm groves were too dangerous to access because often the enemies from whom they hid were other Waorani who would have known the location of the groves. In these times, they substituted the fruits of Oenocarpus bataua for cultivated manioc or peach palm as a source of tepe to supplement the meat diet. In addition, the people enjoyed cacao fruit, and although several species of wild cacao Theobroma spp. occur in the region, the true domesticate, T. cacao, seems only to be found at previously inhabited sites such as these ridgetops.
To understand the highly secretive lifestyle that led to the substitution of nondomesticated fruits for agricultural food, one must appreciate the high levels of internecine violence among Waorani during times of war (e.g., Long Reference Long2019:114–116). Wasserstrom (Reference Wasserstrom2016) correctly rejects the evolutionary-biological explanation given by Beckerman and Yost (Reference Beckerman, Yost, Chacon and Mendoza2007) for Waorani warfare, because it is unlikely that revenge and blood-feuding could have been adaptive without some form of occasional mediation, exogamy, and exchange of people, materials, goods, and ideas. It is also unlikely that being in geographic proximity to the Inca Empire, an area with a long history of endemic conflict, could have influenced Wao patterns of warfare, assuming the Waorani were recent intruders or arrivals in the area (Robarchek and Robarchek Reference Robarchek and Robarchek1998:140–141). Instead, Wao internecine violence (between extended families living on ridgetops) and raids on non-Wao cowode) originated in a more recent period (Cipolletti Reference Cipolletti2002; Long Reference Long2019).
The Waorani as a modern ethnographically identifiable society emerge out of the watershed contact period of the rubber boom. During this period, raids for slaves to work with or for caucheros and the incursions into their lands by Indigenous and non-Indigenous caucheros looking for latex led both to the growing isolation of each small Wao cluster and an increase in the number of daughter groups that were soon seen as strangers by the original group (e.g., Wasserstrom Reference Wasserstrom2016). They may also have led to an increase in deaths from introduced disease to which minimally contacted Indigenous people had little or no herd immunity. Such disease-caused deaths could have exacerbated intragroup killings. Because the Waorani believe all deaths are caused by human agency, spearing those believed to be responsible for those deaths—even if these agents were perceived to be other Waorani—would have been seen as justified; these killings are termed “revenge spearings” (Long Reference Long2019:165). Interpreting these historical factors through Waorani cultural beliefs about grieving and the duty to express it by wreaking violence or revenge, as well as cultural beliefs that the historically contingent increase of sickness and death could be caused spiritually by enemies, resulted in a spiraling increase of internecine violence.
In the three settlements where we worked, every elder had experienced this local violence within his own recent family history. Our older collaborators portray the decades before contact as a period of heightened violence in which groups led by famous warriors such as Guiquita, Moipa, Iteka, and Nihua hid out on ridgetops, from which they could both attack and hide from each other and from interlopers in their habitat. During this period, before fusion and fission became common (Rival Reference Rival2002), one way of gaining wives was for a warrior to kill the father or husband of the woman who would be taken for a wife. Marriage requests were also made during festivities, which were sometimes ended by violence.
To keep their location secret, the Waorani families of this time used fire only during the daytime and avoided going to small streams for water. Instead, they created drinking holes at tiny springs on the sides of the ridges, where trickles of water emerged from the holes of land crabs. According to Nemonte, daughter of the warrior Nihua,
The old people didn't drink the water that runs down the canyons. Now we drink water from the river. . . . But in the old days our grandmothers, our grandfathers drank the water that came out of the holes that the land crabs made. . . . That is why they didn't have stomachaches. They lived well drinking that water. . . . When they saw that water trickled out of a crab hole, they hollowed out the earth so that the water could fill in. And later they scooped up the water with a leaf (cup) and poured it into a large clay pot. . . . They filled (their pots) with those and drank water to live. . . . That's why the grandfathers searched for where the water came out and dug pits there. They bathed with that water. The grandmothers also took water from there to bathe.
This water was then carried back to the ridgetop camps where it was mixed with petome (O. bataua mesocarp) to drink as tepe.
To understand the prevalence of O. bataua on ridgetops occupied by Waorani in the past, it is also necessary to know something about how it was prepared and consumed, as well as its reproductive biology. Petome (the Waorani term for fruits of O. bataua) fruits are small, perhaps twice the size of a large olive, and grow in clusters. Beneath their hard black shell is a thin, edible mesocarp that covers a pit, which is the seed. Because the hard shell of the exocarp must be soaked in warm water before it can be removed, petome fruits were not eaten on the spot. Instead, large numbers of the fruits were gathered in nearby forests, carried back to the camp, and soaked in heated water. Because the water is only slightly heated but not boiled, the seeds of petome fruits tended to remain fertile. After being soaked in the warm water, the hard shells were removed and the mesocarp kneaded into tepe. Children also ate a few of the water-softened fruits as they walked around the camp. They then threw the pits a few yards from their houses, where they sprouted quickly.
This sprouting process creates a significantly different constellation of trees from what happens when petowe (O. bataua) palms bear fruit away from human habitation at lower elevations. In those cases, many of the fruits are swallowed whole by toucans that then fly away; in the absence of humans, toucans and related species (Ramphastidae) are by far the principal dispersal agents of this species (e.g., Lamperty et al. Reference Lamperty, Karubian and Dunham2021). After the fruit is semi-digested, toucans and aracaris regurgitate the pits at a considerable distance from the mother tree, where they may sprout and grow to maturity: this process exemplifies destination-based dispersal (Karubian et al. Reference Karubian, Sork, Roorda, Durães and Smith2010). Other fruits fall by gravity and are dispersed at the base of the mother tree. Although many cotyledons sprout quickly there, the root chemistry of the mother tree eventually kills off the seedlings and prevents them from maturing. In the absence of human habitation, therefore, the mature trees tend to grow much farther apart than what we found on the ridgetop sites. Wao men only cut down these palms (with stone axes) that they planned to use in house construction; when their objective was to gather the fruits, they climbed the trees instead of felling them. Waorani today transplant seedlings away from the mother palm to encourage their growth.
The ridgetops themselves are isolating landscapes. Although they were evidently inhabited for long periods of time, they did not constitute villages per se. In terms of size, they were more like camps of simple foragers; the settlement group could be considered a semi-sedentary camp. The Waorani were not simple hunter-gatherers, however, given their domesticates, including cacao (T. cacao) and some minor horticulture (Rival Reference Rival2002, 2015; Up de Graff, Reference Up de Graff1921; Zurita-Benavides Reference Zurita-Benavides2017). It is in such landscapes—ridgetop forests that were settled by Waorani within the last 125 years or so but have not been occupied since about 1960—that we carried out three of the four forest inventories reported here (Supplemental Appendices 1–4).
Methodology
The objective of the investigation was to determine the extent to which, if any, the human occupants in the current era affected the distribution of tropical rainforest species, mostly on the alpha (or local) level of species diversity (e.g., Balée Reference Balée2013). We used a new approach to studying the impact of recently contacted or still isolated groups in the Upper Amazonian forests. The evidence thus far suggests Indigenous utilization of the tropical forest over a relatively brief historical time of 100–125 years. Peaceful contact only occurred after the 1950s. Today, some Waorani remain out of contact with the members of the nation-state in which they live and are not even in contact with Indigenous persons who speak their own language or dialect.
Because of the features of Upper Amazonian geography and culture, we were not able to use some of the methods used to distinguish anthropogenic forests in the Central or Lower Amazon. In Central and Eastern Amazonia, for example, the presence of terra preta, an Amazon Dark Earth (ADE) containing phosphate and other human-indicator compounds, is a fertile vestige that indicates conscious human management of the soil (e.g., Heckenberger et al. Reference Heckenberger, Christian Russell, Fausto, Toney, Schmidt, Pereira, Franchetto and Kuikuro2008; Woods and McCann Reference Woods and McCann1999). In the case of the Nushiño and the Napo generally, one does not encounter ADE because farming people lived on islands that were already made extremely fertile by the volcanism of the nearby Andes—and the Napo and its tributaries are, in fact, whitewater rivers that drain volcanic sediment directly from the Andes. ADE becomes more important the more downriver on the Amazon one descends (Woods and Denevan Reference Woods, Denevan, Woods, Teixeira, Lehmann, Steiner, WinklerPrins and Rebellato2009).
In the absence of terra preta, our method for selecting where to find and study the precontact human impact began with living Wao people who could tell us about the recent past (i.e., considering 50–60 years to be “recent”) and how their immediate predecessors lived in and used the landscape available to them. Although this research could not have been completed with those Wao Tededo speakers who refuse interaction with external society or its agents (including scientists and researchers, such as us), we were able to interview people who lived before contact and who clearly remember life on the ridgetops. It is important to know this oral history from the Waorani point of view, so that we could combine it with the written history we do have access to, starting with the rubber cycle of the late nineteenth century. The business of rubber extraction was unable to penetrate the Waorani domain south of the Napo (we saw untapped rubber treesFootnote 2 as proof in the Curaray/Nushiño River valleys; Figure 1) because of the violence that would ensue: the Wao speared any intruders into their riverine domain. It was not only the threat of being speared in the open, however, that discouraged rubber tappers and would-be slavers: the Waorani were hidden away from the riverbanks of these small rivers.
The Waorani people who were alive before contact took us to these ridgetops where their grandparents or ancestors had lived. We observed and studied four of these ridgetops that together are roughly one-quarter of a hectare in size. These ridgetops were difficult to access, requiring nearly 50-degree angle climbs over a trail of about 1 km up to relatively small, flat occupation spaces that each were only about 2,500 m2.
A forest inventory can reveal the anthropogenic influences on a landscape. In the Amazonian context, a forest inventory involves collecting all species greater than or equal to 10 cm in diameter at 1.3 m up from the ground level (called DBH) and then determining the frequency, density, and dominance of known disturbance indicators (e.g., Anderson and Posey Reference Anderson and Posey1989; Balée and Campbell Reference Balée and Campbell1990; Peters et al. Reference Peters, Balick, Kahn and Anderson1989). The usual size of the forest inventory in Amazonia is 1 ha (ter Steege et al. Reference Ter Steege, Pitman, Sabatier, Baratolo, Salomão, Guevara and Phillips2013), although up to 3 ha were sometimes inventoried in the past to show asymptotic species/area curves: the amount of area needed in an alpha-type zone to accommodate the maximum or near-maximum number of species. It has also been suggested that most diversity can be sampled with highly linear inventories, such as 10 × 1,000 m or 10 × 3,000 m (e.g., Campbell et al. Reference Campbell, Daly, Prance and Maciel1986).
Because of the small size of the ridgetops—the small mesas or tablelands that coalign with river shapes—and their location between rivers that come close to converging, as do the Curaray and the Nushiño at one point, we could not carry out 1 ha inventories, which as noted earlier are more or less the standard in Amazon forest inventories (e.g., ter Steege et al. Reference Ter Steege, Pitman, Sabatier, Baratolo, Salomão, Guevara and Phillips2013). Instead, taking the ridgetop to be a forest of its own kind and always limited in extent, we took three inventories of 0.20 ha each, or 2,000 m2, at each site. We compared the findings from each of these inventories to a control site, on which the elders told us that the Waorani would not have had villages or any kind of small-scale settlement. The ground of the control site is still well drained, even though it is on a slope between the river and the ridgetop. We divided it into 20 subplots of 10 × 10 m. The four inventories were carried out in the environs of three small Wao settlements—Tepapare, Geyepade, and Gomatan; at the last site one ridgetop and the control site inventories were conducted (Figure 1; Supplemental Appendices 1–4). Voucher specimens are deposited at the Amazonian herbarium of Ecuador (ECUAMZ).
Although we did not make test pits or do other kinds of excavations on any ridgetop, we did find potsherds at two of them. Remarkably, potsherds were also found on the edge of the control site, near a drop-off to a tiny tributary of the Nushiño (Figure 2), even though our Wao collaborators told us that no one had ever lived there. The broken ceramics were evidence of where women in the past, having carried on their shoulders heavy water-filled urns from the tiny tributary far below to the top of the ravine (next to the control site), accidentally dropped and broke them. That is prima facie evidence that these ridgetops were occupied for defensive reasons, for why else would people live where potable water was so difficult to access?

Figure 2. Potsherds found on the surface near the edge of the control site. Photo by William Balée. (Color online)
Results
Table 1 shows the 20 most-dominant species from each of the four study sites. It is noteworthy that, on the three ridgetops, cacao and O. bataua occur among these dominants, but these two species were not found at the control site. These two species also account for between 14% and 24% of the entire basal area of the four sites and between 20% and 30% of the entire basal area of the 20 most-dominant species (Table 2).
Table 1. Twenty Most-Dominant Species in Rank Order of Basal Area (Dominance) from each of the Four Study Sites.

Table 2. Dominance Values of Theobroma cacao and Oenocarpus bataua at the Four Sites.

The basal areas are large and as expected for high rainforests of the Upper Amazon (e.g., Pires and Prance Reference Pires, Prance, Prance and Lovejoy1985), as is the very high species diversity per unit area: almost every other tree is a different species (Gentry Reference Gentry1988). This is the most speciose region of the Amazon Basin because of its environmental gradients, including rainfall, latitude, solar radiation, and probably (young) geological age (all species are ranked by dominance by each inventory in the Supplemental Material). Only two species other than T. cacao and O. bataua are found on two or more of the ridgetop forests (but not the control site; Table 3). In fact, it is the presence of these two extremely important economic species, T. cacao and O. bataua, that demonstrates human impact on the alpha diversity on what is supposedly the most pristine forest of Amazonia.
Table 3. Species among the 20 Most-Dominant Species that Occur in Two or More Anthropogenic Forests but Not the Control Site (Gomatan 02).

These results also show that the species that are found both on the control site (Gomatan 02) and the other three sites are not significantly economic or culturally important (Supplemental Table 1), with the exception of Pourouma cecropiifolia Mart., which has an edible grape-like fruit and has been considered a domesticate (Pedrosa et al. Reference Pedrosa, Clement and Schiett2018). Its presence at Gomatan 02 (control site) may be the result of a dispersal or may reflect the original distribution of the species (cf. Clement et al. Reference Clement, de Cristo-Araújo, D'Eeckenbrugge, Pereira and Picanço-Rodrigues2010; Pedrosa et al. Reference Pedrosa, Clement and Schiett2018). These forests are otherwise all very different from each other (Supplemental Table 2), sharing low proportions of common species. What this means is that ridgetop forests have been manipulated by the Waorani over at least the last 125 years and that these forests—widely and historically considered to be among the most untouched Amazonian rainforests known—are in fact anthropogenic or cultural forests.
Discussion
The ironic question by Cabodevilla (Reference Cabodevilla1994:13), if unanswerable as originally posed, asks whether the Waorani exhibit a period in antiquity, or instead, do they—the Wao people—represent such a time period itself. Cabodevilla's question implicitly addresses the dividing line between antiquity and modernity or between archaeology and ethnography. What this article suggests is that the Waorani do have a history—one embedded in landscapes; namely, ridgetop forests that are not pristine. These landscapes represent vegetation cover managed by the Waorani and their ancestors themselves over about the last 125 years.
What is clear from our inventory fieldwork is that the Waorani occupied and managed ridgetop forests in the central Ecuadorian Amazon between the Napo and the Pastaza at least since the end of the nineteenth century, when they are first mentioned—by their correct ethnonym or not—in the literature (High Reference High2015; Rival Reference Rival2002, Reference Rival2016; Wasserstrom Reference Wasserstrom2016; Zurita-Benavides Reference Zurita-Benavides2014). The question then becomes whether history, in the broadest sense of the term as used in historical ecology (e.g., Crumley et al. Reference Crumley, Lennartsson and Westin2018), can include a hypothetical time before documentation (as in historiographically defined history and ethnohistory), or vice versa, because the logic of the answer does not depend on the subject and predicate order of relations. Another way of asking the question is whether antiquity can be dated to the cultural origins of a place-making people, even if those origins seem recent. We believe that the landscape is key to the answer. One can adduce, as we have here, the historical time period with bookended watershed events from which the Waorani are definitively first known as a distinctive society with a known territory; that is, much of the forested region south of the Napo River. The first period begins with the terminal rubber cycle from about 1885 to 1890 and ends around 1914–1920. For those Waorani who entered into more or less peaceful permanent contact and their descendants, the second period would be from about 1920 to 1960. The bookend for the last period is not yet in place, because of those Tagædi and Taadomenani Wao subgroups who inferentially refuse to comply with or surrender to civilization.
Although we did not create any test pits on the sites, we did find a surface stone ax-head at the ridgetop near the village of Gomatan (Figure 3). Wao people claim to find these in riverbeds and then use them to clear gardens but not to have manufactured them (Cabodevilla Reference Cabodevilla1994:13; Robarchek and Robarchek Reference Robarchek and Robarchek1998:77; Yost Reference Yost and Whitten1981:98). The context of the stone ax-head we found is indicative nevertheless of land management by the Indigenous people in the last 100 or so years. Thus, exactly where human influence is thought to have been negligible on the alpha (and beta) distribution of species—the Upper Amazon, including the Napo and its ridgetop forests—we found artifactual evidence to the contrary.

Figure 3. Wao female elder, Omanca Enqueri, holds the stone ax-head found on the surface at the Gomatan ridgetop, where the photo was taken; she sits at the base of a cacao tree (Theobroma cacao L.). Note the numerous stems, a characteristic of this species. The profusion of stems maximizes the number of cauliflorous fruits that can ripen on a single organism, a feature not seen in other Theobroma species and hence suggestive of deep-time domestication. Photo by Tod Swanson. (Color online)
Therefore, the original Waorani, as known to the documentary record, can be understood in terms of their interaction with and management of the landscape. The human management signatures that we registered on the ridgetop forest composition correspond to the Amazonian rubber cycle or boom in the period from 1850 to 1914—essentially the time frame in which the Wao first become known as a people who violently rejected any contact with outsiders. To clear gardens, they used stone tools made in the ancient past, perhaps thousands of years ago, whenever they did not have raided steel tools on hand (Wasserstrom Reference Wasserstrom2016). They favored tree crops like Oenocarpus bataua and Theobroma cacao on ridgetops. These domesticates and other crops are native to the region and have ancestries predating contact by thousands of years.
The Waorani ancestors also made use of natural resources by relocating them from the forests below the ridgetops to the ridgetops themselves, where they matured into adult ungurahua (petowe) palms and cacao trees (boginka). The distribution (density) and frequency patterns of O. bataua and T. cacao on the ridgetops are most economically explained by previous human habitation. These two species were dominant on all three ridgetop forests studied while not appearing once on the control site. The presence of these species is, in conclusion, a persuasive if unexpected signature of past Waorani management of tablelands high above fast-flowing tributaries south of the Napo River. Therefore, the ridgetop forests cannot a priori be considered to be pristine, just as the Waorani themselves are not savages “Aucas” and indeed never were. They are instead landscape managers—of ridgetop forests.
Acknowledgments
We appreciate the three anonymous peer reviewers for their constructive comments on how to improve this article. In the field, thanks are due to students of IKIAM who helped with measuring trees and laying out transects in three field sites: Angel Crespo, Cielo Montenegro, and Mathew Tello. We also acknowledge Elizabeth Swanson Andi for field assistance and these Indigenous collaborators and their families in the three settlements near where forest inventories were carried out: Cabé Ima, Patricia Irumenga, Wampi Mayancha, Andrés Chimbo, Tanya Mayancha, Omanca Enqueri, Ore Nenquimo, Guiqui Boyotaim, and Betty (Ahuani) Nihua. Diana Chavez Vargas helped with logistics and accounting through the Fundación Cotococha.
Funding Statement
We are grateful to the National Geographic Society for its financial support of project NGS-58639C-19.
Data Availability Statement
The plant specimens collected for the identifications listed in the text, tables, and Supplemental Material are housed in the herbarium city of Puyo, Ecuador: Herbario Amazónico del Ecuador ECUAMZ. These may be found in association with the collectors’ names and inventory numbers of individual trees and lianas on the four forest plots in the study.
Competing Interests
The authors declare none.
Supplemental Material
For supplemental material accompanying this article, visit https://doi.org/10.1017/laq.2022.94.
Supplemental Appendix 1. Ridgetop forest inventory near Gomatan village, ranked in order of dominance (basal area).
Supplemental Appendix 2. Control site forest inventory near Gomatan village, ranked in order of dominance (basal area).
Supplemental Appendix 3. Ridgetop forest inventory near Tepapare village, ranked in order of dominance (basal area).
Supplemental Appendix 4. Ridgetop forest inventory near Geyapare village, ranked in order of dominance (basal area).
Supplemental Table 1. Species shared between the control site (Gomatan 02) and any of the other study sites from among the 20 most-dominant species.
Supplemental Table 2. Jaccard coefficients of similarity between the six pairs of study sites.








