In addition to cognitive testing, reports of subjective cognitive decline (SCD) and of the degree of difficulties performing instrumental activities of daily living (iADLs) ascribed to cognitive decline are core components of clinical neuropsychological assessments in neurodegenerative diseases. Notably, these elements are needed to diagnose Parkinson’s disease (PD) mild cognitive impairment (MCI; Litvan et al., Reference Litvan, Goldman, Tröster, Schmand, Weintraub, Petersen, Mollenhauer, Adler, Marder, Williams-Gray, Aarsland, Kulisevsky, Rodriguez-Oroz, Burn, Barker and Emre2012) or PD dementia (PDD; Emre et al., Reference Emre, Aarsland, Brown, Burn, Duyckaerts, Mizuno, Broe, Cummings, Dickson, Gauthier, Goldman, Goetz, Korczyn, Lees, Levy, Litvan, McKeith, Olanow, Poewe and Dubois2007). SCD, even in the absence of difficulties on performance-based tasks, is also a risk factor for future cognitive decline in PD (Galtier et al., Reference Galtier, Nieto, Lorenzo and Barroso2019; Purri et al., Reference Purri, Brennan, Rick, Xie, Deck, Chahine, Dahodwala, Chen-Plotkin, Duda, Morley, Akhtar, Trojanowski, Siderowf and Weintraub2020). To our knowledge, no studies have investigated specific relationships between cultural diversity and the subjective elements of neuropsychological assessments in patients with PD, and specifically in immigrants. While our previous studies (Statucka et al., Reference Statucka, Cherian, Fasano, Munhoz and Cohn2021; Statucka & Cohn, Reference Statucka and Cohn2019) revealed no significant differences between PD patients born in Anglosphere countries (i.e., Canada, the USA, and the UK) and immigrant patients born elsewhere (International group) in the presence of SCD broadly defined, we did not examine the types and pervasiveness of SCD reported or of iADL difficulties related to cognitive versus physical symptoms.
There is a significant dearth of published studies investigating disparities in subjective cognitive changes in immigrants. However, the literature comparing racial groups within specific societies or residents of different world regions suggests that culture and subjective appraisal of cognition and function may be related. Findings are mixed with respect to difficulties with iADLs (Hackett et al., Reference Hackett, Mis, Drabick and Giovannetti2020; Tappen et al., Reference Tappen, Rosselli and Engstrom2010), and SCD (Borelli et al., Reference Borelli, Zimmer, Bieger, Coelho, Pascoal, Chaves, Amariglio and Castilhos2022; Casillas et al., Reference Casillas, Liang, Vassar and Brown2019; Garcia et al., Reference Garcia, Warner, Garcia, Downer and Raji2021; Jackson et al., Reference Jackson, Rentz, Aghjayan, Buckley, Meneide, Sperling and Amariglio2017; Jang et al., Reference Jang, Choi, Franco, Park, Chiriboga and Kim2022; Nakhla et al., Reference Nakhla, Cohen, Salmon, Smirnov, Marquine, Moore, Schiehser and Zlatar2021; Pluim et al., Reference Pluim, Anzai, Martinez, Munera, Garza-Naveda, Vila-Castelar, Guzmán-Vélez, Ramirez-Gomez, Bustin, Serrano, Babulal, Okada de Oliveira and Quiroz2023; Spitzer & Weber, Reference Spitzer and Weber2019; Tolea et al., Reference Tolea, Chrisphonte and Galvin2020; Wu, Reference Wu2016). This variability in findings may be related to the use of various assessment approaches, disparities in study recruitment or access to care, and group differences in characteristics other than culture that may also contribute to SCD such as education, socioeconomic status, and depression (Borelli et al., Reference Borelli, Zimmer, Bieger, Coelho, Pascoal, Chaves, Amariglio and Castilhos2022; Jang et al., Reference Jang, Choi, Franco, Park, Chiriboga and Kim2022; Lee et al., Reference Lee, Nam, Yi, Bhimla, Nelson and Ma2021; Rodriguez et al., Reference Rodriguez, Ayers, Weiss and Verghese2021; Spitzer & Weber, Reference Spitzer and Weber2019). Therefore, it is difficult to ascertain whether culture or these social and health factors best explain differences in self-reported cognitive changes because these factors are often confounded.
While we recognize the importance of research investigating differences between racial groups, existing findings are not readily applicable when providing neuropsychology services in large, urban, multicultural settings where a key contributor to diversity is immigration. Toronto, Canada, is a good example of this challenge as 47% of residents are immigrants (Statistics Canada, 2021). Health and social disparities may be less pronounced in this group relative to racial groups in the USA, which make up the bulk of the literature on diversity and SCD. Notably, 52% of Toronto’s immigrants obtained permanent resident status under the ‘‘economic’’ category, which favors skilled-workers and professionals who are highly educated (Statistics Canada, 2021). Immigrants are also healthier than Canadian-born individuals based on rates of mortality (Ng, Reference Ng2011), chronic conditions such as cardiovascular conditions (Lu & Ng, Reference Lu and Ng2019; Newbold & Filice, Reference Newbold and Filice2006), and mental health conditions such as depression (Streiner et al., Reference Streiner, Cairney and Veldhuizen2006), although this “healthy immigrant effect” is reduced in the years following immigration.
In the present study, we examine SCD and reported iADL difficulties in Anglosphere and International (immigrant) PD groups in two ways. First, we reviewed the descriptions of cognitive change and functional ability in clinical neuropsychological reports, which summarized changes described during open-ended, semi-structured clinical interviews. A clinical interview is the gold-standard method for soliciting cognitive and functional changes in neuropsychological practice but is seldom examined empirically. We also reviewed responses provided by patients and their care-partners on standardized questionnaires focusing on SCD in the domain of executive functioning and on iADLs. Our aims are to examine potential differences between Anglosphere and International groups in terms of the presence of general and cognitive domain-specific subjective decline as well as the pervasiveness of SCD as defined by the number of total and domain-specific changes reported. If group differences exist, we will investigate whether specific items within relevant cognitive domains drive these differences. We will also examine group differences in self- and family-rated executive dysfunction on a standardized questionnaire. In a similar vein, we will also examine presence and pervasiveness of iADL difficulties reported during the clinical interview and on a standardized questionnaire, as well as the degree to which cognitive and motor symptoms contribute to these difficulties based on questionnaire responses. We also examine whether group differences in other characteristics (e.g., depression severity) contribute to differences in SCD and iADLs. We hypothesized that SCD, broadly defined, may not differ between groups based on our previous work on an overlapping sample, but it is unclear whether their type (i.e., specific cognitive domains) and pervasiveness will differ and whether differences will be observed in iADLs.
Methods
Participants
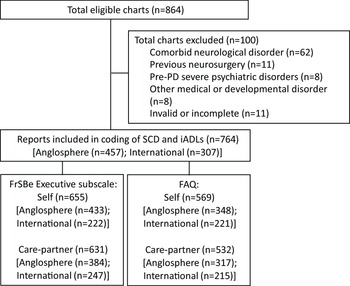
We conducted a chart review of advanced PD patients who completed neuropsychological assessments to determine their candidacy for Deep Brain Stimulation (DBS) at Toronto Western Hospital, University Health Network (UHN) between January 2009 and September 2020. For context, neuropsychological assessments are required for all PD patients considered for DBS to treat their motor symptoms based on clinical guidelines (Lang et al., Reference Lang, Houeto, Krack, Kubu, Lyons, Moro, Ondo, Pahwa, Poewe, Tröster, Uitti and Voon2006), regardless of whether patients report cognitive changes. This study was completed in accordance with the Helsinki Declaration and was approved by the Research Ethics Board at UHN. Exclusion criteria include history of other neurological conditions (e.g., stroke, seizures, moderate/severe traumatic brain injury, neurodevelopment disorders), severe mental illness (e.g., schizophrenia, personality disorders, substance abuse) other than depression or anxiety, and prior brain procedures. A flow diagram depicting included and excluded patients is presented in Figure 1.

Figure 1. Flow diagram of the study and reasons for exclusion.
Of the 764 patients included, 457 were born in Canada, the USA, and the UK (Anglosphere group), and 307 were born outside these countries (International group). Most participants in the latter group were born in Asia (n = 164), followed by Europe (n = 89), the Americas/Caribbean (n = 38), and Africa (n = 16), and none were born in Oceania.
Measures
Demographic and disease-related variables
Demographic variables include age, sex, years of education, level of education [i.e., less than high school (1), high school (2), some post-secondary or college diploma (3), Bachelor’s and more advanced university degrees (4)]. Education level, rather than years of education, is used as our primary measure to minimize the influence of regional differences in educational systems. English fluency is characterized by whether the patient is a native English-speaker, English-as-a-second language but fluent (required no interpreter), has conversational skills in English but an interpreter provided clarifications and assisted with the administration of some tasks, or whether the patient is non-fluent in English (i.e., interpreter assisted with all aspects of the assessment). Of note, interpretation services are offered to patients who did not complete any schooling in English and/or who request them. Interpreters assisted with mood, SCD and iADL questionnaires’ administration, unless adaptations validated in the patient’s language were available. For the International group, years since immigrating was included. We also coded the identity of the care-partner(s) who was present during the clinical interview and completed questionnaires (none, spouse, adult child, other).
Indicators of PD severity include PD duration, levodopa equivalent daily dose (LEDD), and motor scores on the Unified Parkinson’s Disease Rating Scale (UPDRS part 3; Fahn & Elton, Reference Fahn and Elton1987) in the ON state. The MDS-UPDRS part 3 (Goetz et al., Reference Goetz, Tilley, Shaftman, Stebbins, Fahn, Martinez-Martin, Poewe, Sampaio, Stern, Dodel, Dubois, Holloway, Jankovic, Kulisevsky, Lang, Lees, Leurgans, LeWitt, Nyenhuis and LaPelle2008) was used in a subgroup (Anglosphere: n = 88, International: n = 85), and scores were transformed to be equivalent to the older version (Hentz et al., Reference Hentz, Mehta, Shill, Driver-Dunckley, Beach and Adler2015). Higher values on these measures reflect greater disease severity. Depressive symptom severity is coded as normal (0), mild-to-moderate (1) or severe (2) based on scores on the Beck Depression Scale-II [(BDI-II; Beck et al., Reference Beck, Steer and Brown1996): Anglosphere: n = 344, International: n = 238], Geriatric Depression Scale [(GDS; Yesavage et al., Reference Yesavage, Brink, Rose, Lum, Huang, Adey and Leirer1982): Anglosphere: n = 104, International: n = 53], or the UPDRS item 1.3 (Anglosphere: n = 9, International: n = 16).
Subjective cognitive decline
Clinical interview/report
The presence and pervasiveness of SCD were coded based on the descriptions of cognitive changes included in clinical neuropsychological reports. These excerpts summarize SCD reported during a semi-structured clinical interview conducted with each patient alone or jointly with their care-partner(s). The same in-house interview was used by all clinical neuropsychologists who conducted assessments on the DBS service. In this interview, cognitive changes are prompted with a gradual degree of specificity ranging from general (changes in thinking abilities), to domain general (memory), to specific queries (memory for conversations). Domains sampled include memory, attention, executive functions, and language. Domain general prompts were not provided for executive functions as the term is unfamiliar to most. Direct queries of visuospatial/perceptual skills are not included, but changes are captured in spontaneous reports following general prompts.
Coding of extracted passages was completed by two independent raters. The coding scheme was devised by first listing all prompts from the semi-structured interview, including general, broad cognitive domains, and specific queries. Modifications were made following an iterative approach, according to guidelines for narrative data coding (Syed & Nelson, Reference Syed and Nelson2015). First, raters coded 50 patient passages onto our initial items in a binary fashion: “yes” when a decline or difficulty was reported and “no” when an item was described as unchanged or intact or when it was not mentioned. The full excerpt for each patient was coded prior to proceeding to the next patient, and uncoded statements and those that were confusing to raters were compiled. The coding scheme was revised based on these uncoded/confusing statements and on interim inter-rater reliability analyses. Coding was then completed sequentially across a subset of items (e.g., all memory items) for all patients prior to coding another subset, and excerpts were reviewed to ensure that all SCD were captured. Unclassified elements were reviewed, and discrepancies between raters were resolved during consensus meetings. Following completion of coding, some items were merged based on inter-rater reliability analyses and review of frequencies.
A total of 26 items were coded, including six memory items (i.e., general, misplacing objects, event memory, conversation memory, prospective memory, and other), seven attention items (i.e., general, processing speed, tracking conversation, train of thought, sustained attention, distractibility, and attention while reading), eight executive function items (i.e., general, multi-tasking, organization, planning, decision-making, problem-solving, working memory, and other), three language items (i.e., name retrieval, word finding, and expressing oneself), and two items not assigned to these cognitive domains (i.e., visuospatial abilities, fogginess/confusion). Data on these 26 items were combined to derive our main variables including the presence of any SCD and within each of the four cognitive domains, as well as the total number of SCD overall and within each cognitive domain.
Questionnaire
A subset of patients and their care-partners completed the self- and family-rating versions of the Frontal Systems Behavior Scale (FrSBe; Grace & Malloy, Reference Grace and Malloy2001). The frequency of each behaviour is rated on a five-point Likert scale for two time periods: (1) prior to disease onset (pre-PD) and (2) currently. We report data only for the Executive Dysfunction subscale (17 items), including pre-PD and current ratings, and change in raw scores which is our primary SCD measure.
Of the full sample, 655 patients (Anglosphere: n = 433, International: n = 222) completed the self-rating form, and 631 care-partners (Anglosphere: n = 384, International: n = 247) completed the family-rating form. Missing data are not random as patients who are functioning relatively well physically and cognitively are more likely to be unaccompanied during their assessment, and patients who proceed through testing slowly due to severe physical or cognitive symptoms, or due to the need for interpretation services may run out of time or stamina to complete questionnaires.
Instrumental activities of daily living
Clinical interview/report
The presence of iADL difficulties was coded based on excerpts from the clinical neuropsychological reports which summarize information obtained during a semi-structured interview. We used the same iterative coding approach as described for SCD. A code of “yes” was assigned to items for which a change or difficulty was endorsed regardless of the reason, and “no” when a change or difficulty was explicitly denied or when the item was not mentioned. Nine items were coded: medication, appointments, chores, cooking, finances, shopping, transportation, work, and hobbies. The presence of changes or difficulties with specific iADLs and the total number of items endorsed were tallied.
Questionnaire
A subset of patients and their care-partners completed the FAQ (Pfeffer et al., Reference Pfeffer, Kurosaki, Harrah, Chance and Filos1982), which includes ten items reflecting daily activities. The patient’s ability to perform these activities is rated as normal or never did (0), has difficulty but does by self (1), requires assistance (2), or dependent (3). The ten items are summed to reflect the degree of iADL difficulties. As part of an in-house adaptation, for each item rated ≥1, patients and their care-partners rated the degree to which motor and cognitive symptoms limit the patient’s ability to perform the activity [not at all (0), mildly (1), moderately (2), severely (3)]. Scores were tallied into motor and cognitive subscales.
Of the full sample, 569 patients (Anglosphere: n = 348, International: n = 221) completed the self-report form, and 532 care-partners (Anglosphere: n = 317, International: n = 215) completed the informant form. Missing data are not random for the same reasons described above, but in addition, the FAQ is available only for assessments conducted since mid-2012.
Statistical analysis
Group differences in disease-related and socio-demographic variables were examined with the Mann-Whitney U test and chi-square. To address whether the presence and type of SCD differ between Anglosphere and International patients, we first compared the frequency of SCD in general and specific cognitive domains (memory, attention, executive functions, and language) coded from clinical reports between groups using chi-square tests. Second, to examine pervasiveness of SCD, we used Mann-Whitney U test to compare groups based on the total number of SCD overall and within each of four cognitive domains reported during the clinical interview. Bonferroni corrections were applied to both sets of analyses separately (i.e., p < .05 corrected for five comparisons each). For cognitive domains showing significant group differences in these analyses, we examined the frequency of endorsement of specific items (post-hoc analyses) using chi-square tests with Bonferroni corrections for the significance level threshold (i.e., p < .05 corrected for 11 comparisons). For completeness, frequency of endorsed items from domains showing no difference and related statistics are presented in Supplementary Materials. Third, Mann-Whitney U tests were conducted to examine group differences in the degree of change from pre-PD to currently on the self-rating and family-rating FrSBe executive dysfunction subscale, and for completeness, groups’ ratings at both timepoints. Bonferroni corrections were applied (p < .05 corrected for six comparisons).
To examine group differences in iADLs, we first analyzed the overall presence of reported difficulties (yes/no), as well total number of items endorsed during the clinical interview using chi-square and Mann-Whitney U tests, respectively. For completeness, endorsement rates for individual items are presented in Supplementary Materials. Second, total score on the FAQ (self- and informant-versions), and FAQ motor and cognitive subscores were analyzed with Mann-Whitney U tests, and Bonferroni corrections were applied (p < .05 corrected for six analyses). Because depression severity ratings were more elevated in the International group and may account for greater SCD or iADL difficulties in this group, we repeated some analyses after covarying depression severity using the Quade nonparametric ANCOVA based on ranks. Effect sizes (Cohen’s d and OR) are also presented. All analyses were conducted using SPSS v22.
Results
Disease-related and demographic characteristics
Demographic and disease-related data, and related statistics and effect sizes, are presented for the full sample in Table 1, and for the subsets of participants included in FrSBe and FAQ analyses in Supplementary Materials. Across these samples, there are no significant differences between the Anglosphere and International groups in terms of age, sex, and level of education, as well as across indicators of disease severity. The proportion of individuals for whom English is their first language is markedly higher in the Anglosphere group (OR = 100.42). As for the identity of the care-partner(s), the International group is more likely to have an adult child and less likely to have a spouse present. Lastly, the International group’s depression severity ratings are higher than the Anglosphere group’s, although the magnitude of this effect is small (d = 0.19).
Table 1. Characteristics of Anglosphere and International PD groups (Frequency and Mean [SD])

a 1 = less than high school, 2 = high school, 3 = some post-secondary or college diploma, 4 = Bachelor’s and more advanced university degrees.
b UPDRS, Unified Parkinson’s Disease Rating Scale; LEDD, levodopa equivalent daily dose.
c Clinical interviews conducted with spouse and those with child overlapped in that 3.3% of the Anglosphere group and 14.1% of the International group had both present.
Subjective cognitive decline (SCD)
Clinical interview/report
As shown in Table 2, the groups show no differences in the presence of SCD or number of items endorsed overall and in the memory and attention domains. However, both the presence and number of items endorsed are greater in the Anglosphere group relative to the International group in the domains of executive functioning and language. Post-hoc analyses of specific items revealed that the Anglosphere group was more likely to report changes in planning and word finding. These effects are small in magnitude (d range: 0.24–0.25; OR range: 1.53–1.96).
Table 2. Subjective cognitive decline (SCD) in Anglosphere and International groups (Frequency and Mean [SD])

a No “general” or “other” complaints were reported in the Language domain.
* p-value significant after Bonferroni correction.
FrSBe questionnaire
As shown in Table 3, there are no significant group differences in self-rating of executive dysfunction prior to PD and currently, and in the degree of change between these timepoints. Group differences in care-partners’ report of executive dysfunction prior to PD and the degree of changes also do not reach the statistical threshold after corrections for multiple comparisons. However, care-partners of International patients do report greater levels of current executive dysfunction than care-partners of patients from the Anglosphere group (d = 0.29). Group differences remain after entering depression severity ratings as a covariate (F(1, 629) = 7.44, p = .007, d = 0.22).
Table 3. Questionnaires in Anglosphere and International groups (mean, [SD])

a FrSBe, Frontal Systems Behavior Scale; FAQ, Functional Assessment Questionnaire.
* p-value significant after Bonferroni correction.
Instrumental activities of daily living (iADLs)
Clinical interview/report
The groups show no significant differences in the presence of iADL difficulties reported (Anglosphere: 76.8%, International: 74.6%; χ2 = 0.49, p = .48, OR = 1.13, 95%CI [0.81, 1.58]). Similarly, the total number of iADL difficulties reported across nine items did not differ significantly between the Anglosphere group (M = 1.49, SD = 1.25) and the International group (M = 1.58, SD = 1.40; U = 71,727.5, p = .59, d = 0.04).
FAQ Questionnaire
Greater iADL difficulties are reported by the International group relative to the Anglosphere group on the FAQ by patients and care-partners. Regarding the source of these difficulties, the International group report greater limitations related to motor symptoms, but there are no group differences in the degree to which cognitive difficulties limit iADLs. Group differences on the total and motor scores of the FAQ persist after covarying depression severity ratings on the self-rated version [total: F(1, 567) = 10.81, p = .001, d = .28; motor: F(1, 561) = 10.51, p = .001, d = .27] and care-partner versions of the scale [total: F(1, 531) = 14.12, p < .001, d = .33; motor: F(1, 527) = 22.59, p < .001, d = .41].
Discussion
In a large sample of individuals with advanced PD seen consecutively to assess their suitability for DBS surgery in Toronto, Canada, minimal differences are observed between patients born in Anglosphere countries versus immigrants born elsewhere in terms of subjective cognitive decline. Based on information provided during clinical interviews with patients and their care-partners, the presence and pervasiveness of SCD overall and within the domains of memory and attention do not differ significantly between groups. As for iADLs, there are also no group differences in the degree to which cognitive change is perceived as limiting their daily functioning on the FAQ, although the International group reports more difficulties on the FAQ which they ascribed to greater physical limitations rather than to cognitive decline. However, SCD findings within the executive function domain are mixed and contradictory. The Anglosphere group is more likely to report changes in executive functioning and specifically with respect to planning during the interview. In contrast, no group difference is observed on the self-rating version of the FrSBe executive dysfunction scale in terms pre-PD and current executive dysfunction, or in the magnitude of change between these timepoints. Similarly, care-partners pre-PD ratings and degree of change in executive functioning do not differ between groups, although care-partners of patients from the International group rated current executive dysfunction as greater than care-partners of the Anglosphere group. Lastly, the Anglosphere group is more likely to report language decline and specifically word-finding difficulties than the International group during the clinical interview. We discuss these findings and limitations below.
First, the magnitude of all group differences reported is small based on effect size interpretation, and in many cases, below statistical threshold. This contrasts with the medium to large effect sizes found on performance-based tasks such as cognitive screeners (Statucka et al., Reference Statucka, Cherian, Fasano, Munhoz and Cohn2021), and measures of executive functions and visuoperception in an overlapping sample (Statucka & Cohn, Reference Statucka and Cohn2019). This suggests that cultural differences are less pronounced in patients’ own experience of cognitive change than on performance-based measures. Interestingly, null findings in the memory and attention domains are found on both self-reported measures in the current study and performance-based measures in our previous work (Statucka & Cohn, Reference Statucka and Cohn2019), suggesting that they may provide fairer indicators of cognitive decline in immigrants with PD, which is important since these cognitive domains are commonly affected in this disease (Hoogland et al., Reference Hoogland, van Wanrooij, Boel, Goldman, Stebbins, Dalrymple-Alford, Marras, Adler, Junque, Pedersen, Mollenhauer, Zabetian, Eslinger, Lewis, Wu, Klein, Rodriguez-Oroz, Cammisuli and Barone2018).
Second, the contradictory results pertaining to SCD in the executive domain are not surprising and may have been evident in other domains if these had also been assessed in multiple ways. This is consistent with variable findings observed across studies in racialized older adults as a function of the tools used to elicit SCD (Casillas et al., Reference Casillas, Liang, Vassar and Brown2019; versus Pluim et al., Reference Pluim, Anzai, Martinez, Munera, Garza-Naveda, Vila-Castelar, Guzmán-Vélez, Ramirez-Gomez, Bustin, Serrano, Babulal, Okada de Oliveira and Quiroz2023), as well as with poor agreement (κ ≤ 0.29) between three SCD methods within a single PD group (AlDakheel et al., Reference AlDakheel, Gasca-Salas, Armstrong, Duff-Canning and Marras2019). Our discrepant results may relate to format (interview versus questionnaires), type of question (change versus frequency), respondent (self versus care-partner), measurement error, or type I errors. Overall, inconsistent findings in SCD related to executive dysfunction and the previously documented bias on executive performance-based tools may suggest poorer validity within this domain in diverse groups, which presents a problem as executive dysfunction is very common in PD (Hoogland et al., Reference Hoogland, van Wanrooij, Boel, Goldman, Stebbins, Dalrymple-Alford, Marras, Adler, Junque, Pedersen, Mollenhauer, Zabetian, Eslinger, Lewis, Wu, Klein, Rodriguez-Oroz, Cammisuli and Barone2018). Importantly, several executive function items from the clinical interview and the FrSBe self-report show no significant group differences, suggesting that these items and measures may be less prone to cultural differences.
Third, findings related to language abilities are somewhat surprising. Word-finding difficulty is the most frequently endorsed SCD item in both groups (42% and 53%), although performance in language tasks such as naming is typically well-preserved in PD (Hoogland et al., Reference Hoogland, van Wanrooij, Boel, Goldman, Stebbins, Dalrymple-Alford, Marras, Adler, Junque, Pedersen, Mollenhauer, Zabetian, Eslinger, Lewis, Wu, Klein, Rodriguez-Oroz, Cammisuli and Barone2018). As such, these elevated reporting rates may reflect an aging rather than a disease effect. In addition, the increased language SCD rate in the Anglosphere relative to the International group contrasts with evidence showing word-finding difficulties on performance-based tasks to be more common in bilinguals than in monolinguals (Bialystok et al., Reference Bialystok, Craik, Green and Gollan2009), and rates of bilingualism are likely higher in the International group. While we can speculate on several possible reasons for this disparity, we are unaware of any studies investigating SCD in language abilities in bilinguals versus monolinguals or examining the concordance between SCD and performance-based language tasks.
As for iADLs, greater changes in the International relative to the Anglosphere group are observed on both the self and informant FAQ, but not based on information obtained during the clinical interview. Our FAQ findings contrast with another study showing comparable performance in Afro-Caribbean, Hispanic, Black, and White community-dwelling older residents of Florida despite the likely high proportion of immigrants in the former two racial groups (Tappen et al., Reference Tappen, Rosselli and Engstrom2010). In our study, group differences in the FAQ total score are related to the degree to which physical (but not cognitive) symptoms limit iADLs. This is interesting as the two groups do not differ on indicators of disease severity. This group difference and higher FAQ motor relative to cognitive subscores in both groups highlight the importance of isolating physical disability from cognitive decline in PD. While our homegrown adaptation of the FAQ addresses this by including ratings of motor and cognitive limitations, it has not been validated. However, Becker et al. (Reference Becker, Bäumer, Maetzler, Nussbaum, Timmers, Van Nueten, Salvadore, Zaunbrecher, Roeben, Brockmann, Streffer, Berg and Liepelt-Scarfone2020) used regression models to derive motor and cognitive subscores by weighting FAQ items and subsequently showed that the cognitive subscore predicts worse cognitive prognosis 3.78 years later in PD (Becker et al., Reference Becker, Bode, Brockmann, Gasser, Michaelis, Solbrig, Nuerk, Schulte, Maetzler, Zimmermann, Berg and Liepelt-Scarfone2022).
A strength of our study is the coding of data derived from clinical interviews, which is the gold standard in neuropsychology practice that enables patients and their care-partners to describe their experience in their own words, and allows clinicians to ask for elaborations. While culture can color SCD expressions, the absence of group differences in “other” categories suggests such SCD were mapped onto concepts shared across patients. As this is a retrospective study, we did not record, transcribe, and then code transcriptions of the clinical interviews as in a true qualitative study but rather, we relied on written descriptions of this information in the clinical reports as summarized by the neuropsychologist. In the years spanning this study (2009–2020), reports were written by different neuropsychologists whose individual interviewing and writing styles may introduce variations and distortions into our data despite using the same semi-structured interview. Importantly, 75% of the reports used in this study were written by M.C. and M.S, with similar proportions of International patient reports (40% vs 44%), suggesting that differences in style would not affect one group disproportionally. The approach of coding SCD from written reports is laborious and not suitable for inclusion in large clinical trials. However, the detailed reporting of the frequency of endorsed items per group may assist in selecting and designing SCD and iADL tools in studies of cognitive functioning in diverse PD groups.
As for additional limitations, our sample is not fully representative of the Canadian PD population. Patients who are considered for DBS are rarely older than 70 nor present with frank PDD, as these are contraindications for DBS. The latter is particularly problematic to the examination of iADLs as most patients in our study retained independence despite experiencing difficulties. Relatedly, the use of clinical data collected consecutively in the context of a universal health care system may appear less prone to recruitment biases than prospective studies, and the fact that our two groups were being considered for DBS at similar disease stages may give the impression that there are no disparities in accessing DBS in Ontario, Canada. Unfortunately, this is not the case. Crispo and colleagues (Reference Crispo, Lam, Le, Richard, Shariff, Ansell, Squarzolo, Marras, Willis and Seitz2020) demonstrated that PD patients living in Ontario neighborhoods with the highest concentration of visible minorities were less likely to receive DBS than those living in predominantly white neighborhoods.
Second, missing data from questionnaires are not random. Patients with limited English proficiency and those with severe physical and/or cognitive limitations are less likely to complete the self-rated questionnaires, whereas patients who are doing relatively well physically and cognitively are more likely to attend their neuropsychological assessment alone and be missing care-partners’ questionnaires. Importantly, this selection bias does not apply to SCD and iADL information coded from clinical reports, given that all patients completed a semi-structured clinical interview. Third, we compared groups based on the immigration status of patients, but we have no information on the status of care-partners. Fourth, while questionnaire-rated differences in iADL difficulties and executive dysfunction persist after accounting for depressive symptoms, self-rated depression measures are also susceptible to cultural differences (Dere et al., Reference Dere, Watters, Yu, Bagby, Ryder and Harkness2015; Seppanen et al., Reference Seppanen, Lankila, Auvinen, Miettunen, Korpelainen and Timonen2022), and their use as covariates in statistical models may remove part of the effect of interest. Fifth, characteristics that may contribute to SCD and likely differ between groups were not available, notably socioeconomic status (SES). However, given that low SES has been associated with elevated SCD (Rodriguez et al., Reference Rodriguez, Ayers, Weiss and Verghese2021; Tolea et al., Reference Tolea, Chrisphonte and Galvin2020) and is possibly lower in our International group, our findings of greater rates of SCD in the Anglosphere group would remain surprising. Another limitation relates to the interindividual variability in the degree of involvement with iADLs. Several factors are likely contributing to this across patients (e.g., division of responsibilities in their household), and some may be more prominent in the International group. However, we found no group difference in activities wherein limited English fluency can be a barrier (e.g., appointments, finances).
Lastly, we divided our sample into two large subsamples based on the rationale that individuals from the Anglosphere share a common language and originated from countries with similar cultural/historical roots, with high levels of socioeconomic development, and where most neuropsychological tools and neuropsychology practice guidelines are developed. In stark contrast, the International group is much more diverse and includes individuals from 69 countries who identified over 60 different native languages. Therefore, though we found no convincing group differences between our very broad International group and the Anglosphere group in terms of reported SCD and iADL difficulties, such differences may exist in smaller ethnic or racialized groups as demonstrated in other studies (Ganbat & Wu, Reference Ganbat and Wu2021; Lee et al., Reference Lee, Nam, Yi, Bhimla, Nelson and Ma2021). Given the heterogeneity in our sample, such detailed analyses are not feasible and are beyond the scope of the current study.
In conclusion, our study demonstrates that immigration status has limited effect on whether people report cognitive change and the degree to which such change limits their daily functioning. Given the well-documented bias on several performance-based tools, significant weight should be given to patients’ and care-partners’ perspective on their cognition in accordance with diagnostic criteria. This is illustrated in our previous work showing that when SCD is considered together with performance-based data, the rate of PD-MCI is not significantly different between groups (Statucka et al., Reference Statucka, Cherian, Fasano, Munhoz and Cohn2021; Statucka & Cohn, Reference Statucka and Cohn2019). While the importance of SCD may be obvious to clinical neuropsychologists, it is common for PD studies to rely solely on performance-based measures to define PD-related cognitive dysfunction, for instance as part of exclusion criteria, which contributes to the reduced representation of diverse patients in clinical trials. In addition to being relevant to the diagnosis of cognitive dysfunction, multiculturalism may impact prognostication, and therefore, future research is needed to consider diversity in investigating both subjective and performance-based neuropsychological tools as predictors of cognitive outcome with PD progression and following DBS.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S1355617723000668
Acknowledgements
We have no conflict of interest to disclose. This work was supported by The Michael J. Fox Foundation for Parkinson’s Research (grant number MJFF-020819).