Introduction
The Ediacaran Period (635–539 Ma) represents a critical transition in the evolutionary path of the Earth-life system. To better understand the tempo, mode, and mechanisms of Ediacaran evolution, a solid chronostratigraphic framework is needed. In the past two decades, considerable progress has been made toward global chronostratigraphic correlation of Ediacaran strata (Xiao and Narbonne, Reference Xiao, Narbonne, Gradstein, Ogg, Schmitz and Ogg2020). However, key obstacles have yet to be overcome to achieve Phanerozoic-style chronostratigraphic division and correlation based on biostratigraphic data. Importantly, although there has been increasing evidence for a first-order subdivision and correlation of upper Ediacaran strata (ca. 580–539 Ma) on the basis of Ediacara-type macrofossils (Waggoner, Reference Waggoner2003; Boag et al., Reference Boag, Darroch and Laflamme2016; Muscente et al., Reference Muscente, Bykova, Boag, Buatois, Mángano, Eleish, Prabhu, Pan, Meyer, Schiffbauer, Fox, Hazen and Knoll2019), biostratigraphic subdivision and correlation of lower Ediacaran strata (ca. 635–580 Ma) on the basis of microfossils has not been achieved on a global scale. This is a major weakness in Ediacaran evolution and biostratigraphy, not only because microfossils are the foundation to understand early Ediacaran biodiversity and evolution, but also because they have potential as an effective tool for global biostratigraphic correlation (just as they do in Phanerozoic biostratigraphy).
One group of Ediacaran microfossils—variously known as giant acanthomorph acritarchs (Vidal, Reference Vidal1990), Doushantuo-Pertatataka acanthomorphs or DPAs (Zhou et al., Reference Zhou, Brasier and Xue2001, Reference Zhou, Xie, McFadden, Xiao and Yuan2007), Ediacaran complex acanthomorph palynoflora or ECAP (Grey, Reference Grey2005), and large ornamented Ediacaran microfossils or LOEMs (Cohen et al., Reference Cohen, Knoll and Kodner2009)—is particularly useful in biostratigraphic correlation of lower Ediacaran strata. These acanthomorphic acritarchs or spinose organic-walled microfossils are characterized by large spherical vesicles (typically >200 μm in diameter; Xiao et al., Reference Xiao, Zhou, Liu, Wang and Yuan2014) ornamented with morphologically complex processes or spines. They are taxonomically diverse, particularly in the lower Ediacaran system, although large acanthomorphs are sparsely known from older strata (Agić et al., Reference Agić, Moczydłowska and Yin2015) and smaller acanthomorphs (<100 μm in diameter) are also present in the Ediacaran (Yin et al., Reference Yin, Wang, Yuan and Zhou2011).
Earlier work treated Ediacaran acanthomorphs as a coherent group of microfossils that diversified after the ca. 635 Ma Marinoan glaciation and largely disappeared before the ca. 580 Ma Gaskiers glaciation and the Shuram negative δ13C excursion or its equivalent EN3 in South China (Xiao, Reference Xiao, Jenkins, McMenamin, Sohl and McKay2004a; Zhou et al., Reference Zhou, Xie, McFadden, Xiao and Yuan2007; McFadden et al., Reference McFadden, Huang, Chu, Jiang, Kaufman, Zhou, Yuan and Xiao2008). More recent work, however, demonstrated that some acanthomorphs taxa that were thought to be restricted in the lower Ediacaran may range into upper Ediacaran and pre-Ediacaran strata. For example, Ouyang et al. (Reference Ouyang, Guan, Zhou and Xiao2017) argued that some DPA taxa extend into the Shuram (EN3) interval at the Liujiayuanzi section in Hunan Province, South China. Grazhdankin et al. (Reference Grazhdankin, Nagovitsin, Golubkova, Karlova, Kochnev, Rogov and Marusin2020) reported DPA taxa from the lower Cambrian Oppokun Formation at the Khastakhskaya borehole, Lena-Anabar Basin, north-central Siberia, although the Cambrian age interpretation was based on small shelly fossils such as Cambrotubulus Missarzhevsky in Rozanov et al., Reference Rozanov, Missarzhevskii, Volkova, Voronova, Krylov, Keller, Korolyuk, Lendzion, Michniak, Pykhova and Sidarov1969, and Anabarites Missarzhevsky in Voronova and Missarzhevsky, Reference Voronova and Missarzhevsky1969, which have also been found in terminal Ediacaran strata (Knoll et al., Reference Knoll, Grotzinger, Kaufman and Kolosov1995; Zhu et al., Reference Zhu, Zhuravlev, Wood, Zhao and Sukhov2017; Cai et al., Reference Cai, Xiao, Li and Hua2019), and hence these DPAs are best regarded as terminal Ediacaran–lower Cambrian in age. Golubkova et al. (Reference Golubkova, Zaitseva, Kuznetsov, Dovzhikova and Maslov2015) reported DPAs from upper Ediacaran strata at the Keltmen-1 Borehole in the Timan Ridge of the East European Platform, although Vorob'Eva et al. (Reference Vorob'Eva, Sergeev and Knoll2009) considered these strata middle Ediacaran in age. Anderson et al. (Reference Anderson, Macdonald, Jones, McMahon and Briggs2017, Reference Anderson, McMahon, Macdonald, Jones and Briggs2019) described a few DPA taxa from the upper Khesen Formation in the Khuvsgul terrane of northern Mongolia, which is considered terminal Ediacaran but may well be early Cambrian in age (Anttila et al., Reference Anttila, Macdonald and Bold2021). Perhaps the most contentious is the report of numerous DPA taxa, including several eponymous taxa used to define Ediacaran acanthomorph assemblage biozones, from the Semri Group of the Lower Vindhyan Supergroup in the Chambal Valley of eastern Rajasthan of central-western India (Prasad and Asher, Reference Prasad and Asher2016) because the Semri Group in the Son Valley of central India is widely regarded as Paleo-/Mesoproterozoic in age (Rasmussen et al., Reference Rasmussen, Bose, Sarkar, Banerjee, Fletcher and McNaughton2002; Ray et al., Reference Ray, Martin, Veizer and Bowring2002), although Prasad and Asher (Reference Prasad and Asher2016, Reference Prasad and Asher2021) argued this unit is Ediacaran in age. The potential occurrence of DPA taxa in Paleo-/Mesoproterozoic strata would greatly complicate and compromise our attempt to use them to divide and correlate Ediacaran strata, and thus the age and taxonomic identification of these Semri DPA taxa warrant close scrutiny.
On the bright side, there has been success in regional biostratigraphic correlation of lower Ediacaran strata based on acanthomorphic acritarchs. Grey (Reference Grey2005), for example, building upon an earlier study by Zang and Walter (Reference Zang and Walter1992), systematically investigated acanthomorphs from early Ediacaran shales and fine-grained siltstones using the hydrofluoric (HF) extraction method. She established four acanthomorph biozones that can be used to correlate lower Ediacaran strata across the Officer Basin, Amadeus Basin, and Stuart Shelf in Australia. Other paleontologists have applied the HF extraction method to analyze acanthomorphs from lower Ediacaran shales and siltstones in Siberia (Kolosova, Reference Kolosova1991; Moczydłowska, Vidal, and Rudavskaya, Reference Moczydłowska, Vidal and Rudavskaya1993; Golubkova et al., Reference Golubkova, Raevskaya and Kuznetsov2010; Sergeev et al., Reference Sergeev, Knoll and Vorob'Eva2011; Moczydłowska and Nagovitsin, Reference Moczydłowska and Nagovitsin2012) and Baltica (Vorob'Eva et al., Reference Vorob'Eva, Sergeev and Knoll2009; Golubkova et al., Reference Golubkova, Zaitseva, Kuznetsov, Dovzhikova and Maslov2015), although a regional biostratigraphic zonation has not been established.
Silicified and phosphatized acanthomorphs also feature prominently in early Ediacaran biostratigraphy. The preservation of these acanthomorphs involve early diagenetic silica or phosphate precipitation on organic substrates, thus encasing organic substrates (e.g., cell walls) and essentially forming three-dimensional casts and molds of organic structures (e.g., cells) (Xiao and Tang, Reference Xiao and Tang2022). Acanthomorphs preserved in cherts and phosphorites are often studied in thin sections (e.g., Yin and Li, Reference Yin and Li1978) and phosphatized microfossils preserved in a carbonate matrix also can be extracted using the acetic acid maceration method (e.g., Xiao and Knoll, Reference Xiao and Knoll2000). In several studies of silicified acanthomorphs from the lower Ediacaran Doushantuo Formation in the Yangtze Gorges area of South China (McFadden et al., Reference McFadden, Xiao, Zhou and Kowalewski2009; Yin et al., Reference Yin, Liu, Chen, Tang, Gao and Wang2009; Liu et al., Reference Liu, Xiao, Yin, Chen, Zhou and Li2014a; Liu and Moczydłowska, Reference Liu and Moczydłowska2019), different schemes of acanthomorph-based biostratigraphic zonation have been proposed. Although the application of these biozones in regional biostratigraphic correlation remains a challenge, preliminary data indicate that numerous acanthomorphs have robust biostratigraphic significance in the Yangtze Gorges area (Ouyang et al., Reference Ouyang, Zhou, Xiao, Guan, Chen, Yuan and Sun2021). The encouraging success from the Yangtze Gorges area gives us hope that intra- and inter-basinal correlation of lower Ediacaran strata using silicified and phosphatized acanthomorphs is achievable. This optimism is strengthened by a multiplicity of acanthomorphs from Ediacaran cherts and phosphorites in South China (e.g., Xiao et al., Reference Xiao, Zhou, Liu, Wang and Yuan2014; Liu and Moczydłowska, Reference Liu and Moczydłowska2019), northern India (e.g., Shukla and Tiwari, Reference Shukla and Tiwari2014; Joshi and Tiwari, Reference Joshi and Tiwari2016; Sharma et al., Reference Sharma, Shukla and Sergeev2021), Baltica (Vidal, Reference Vidal1990), Svalbard (Knoll, Reference Knoll1992), Greenland (Willman et al., Reference Willman, Peel, Ineson, Schovsbo, Rugen and Frei2021), and Mongolia (Anderson et al., Reference Anderson, Macdonald, Jones, McMahon and Briggs2017, Reference Anderson, McMahon, Macdonald, Jones and Briggs2019).
A necessary step toward a global acanthomorph-based biostratigraphic framework is to test the biozonations from Australia and the Yangtze Gorges area in other sedimentary basins. There are, however, several major obstacles. First, acanthomorphs preserved in shales versus cherts/phosphorites are studied using different methods, may have different taphonomic histories, and may represent different depositional environments. These differences unavoidably make it difficult for a direct comparison between these taphonomic windows; indeed, taxonomic criteria are not practically the same for acanthomorphs preserved in shales versus cherts and phosphorites (Xiao et al., Reference Xiao, Zhou, Liu, Wang and Yuan2014). Second, there is considerable variation from basin to basin in terms of sampling intensity. Among silicified acanthomorph assemblages, for example, those in the Doushantuo Formation in the Yangtze Gorges area have been much more extensively investigated than those in other early Ediacaran basins, with data accumulated over several decades by multiple research groups who sampled dozens of easily accessible localities, examined tens of thousands of thin sections, and detailed their results in numerous monographs (e.g., Yin and Li, Reference Yin and Li1978; Yin, Reference Yin1987; Zhang et al., Reference Zhang, Yin, Xiao and Knoll1998; McFadden et al., Reference McFadden, Xiao, Zhou and Kowalewski2009; Liu et al., Reference Liu, Xiao, Yin, Chen, Zhou and Li2014a; Liu and Moczydłowska, Reference Liu and Moczydłowska2019; Ouyang et al., Reference Ouyang, Zhou, Xiao, Guan, Chen, Yuan and Sun2021). In comparison, silicified Ediacaran acanthomorphs from the Scotia Group in Svalbard (Knoll, Reference Knoll1992) and the Biskopås Conglomerate in southern Norway (Spjeldnaes, Reference Spjeldnaes1963, Reference Spjeldnaes1967; Vidal, Reference Vidal1990) are less extensively surveyed, although those from the Infra-Krol and Krol A formations in the Krol Belt of northern India have gained more research attention in recent years (Shukla and Tiwari, Reference Shukla and Tiwari2014; Joshi and Tiwari, Reference Joshi and Tiwari2016; Sharma et al., Reference Sharma, Shukla and Sergeev2021). This disparity in sampling and research intensity makes it difficult to carry out detailed inter-basinal correlation. Third, other than the Doushantuo Formation in South China (e.g., McFadden et al., Reference McFadden, Huang, Chu, Jiang, Kaufman, Zhou, Yuan and Xiao2008; Xiao et al., Reference Xiao, McFadden, Peek, Kaufman, Zhou, Jiang and Hu2012; Liu et al., Reference Liu, Xiao, Yin, Chen, Zhou and Li2014a; Ouyang et al., Reference Ouyang, Zhou, Xiao, Chen and Shao2019), few Ediacaran successions have been assessed using an integrative approach to calibrate and test acanthomorph biostratigraphy versus δ13C chemostratigraphy.
To address these problems and to achieve a global chronostratigraphic framework for the early Ediacaran Period, we envision the steps outlined below. First, it is imperative to substantially improve the sampling intensity of under-studied successions. Second, to isolate taphonomic factors as a potential source of bias, it is necessary to carry out comparative studies of acanthomorph assemblages preserved in similar taphonomic mode. Third, after biozonation has been established and tested among assemblages of similar taphonomic mode, we need to bridge the gap between the silicification/phosphatization and carbonaceous-compression modes by comparing acanthomorphs from chert/phosphorite and shale facies. It is important to emphasize that acanthomorph biostratigraphic data, whenever possible, must be integrated with other chronostratigraphic tools such as δ13C, 87Sr/87Sr, and geochronometric dates (Xiao et al., Reference Xiao, Narbonne, Zhou, Laflamme, Grazhdankin, Moczydłowska-Vidal and Cui2016), as has been done in the Doushantuo Formation in South China (e.g., McFadden et al., Reference McFadden, Huang, Chu, Jiang, Kaufman, Zhou, Yuan and Xiao2008; Liu et al., Reference Liu, Xiao, Yin, Chen, Zhou and Li2014a; Ouyang et al., Reference Ouyang, Guan, Zhou and Xiao2017, Reference Ouyang, Zhou, Xiao, Chen and Shao2019; Liu and Moczydłowska, Reference Liu and Moczydłowska2019).
As an effort to implement this campaign, we carried out an integrative study of the Krol A Formation in the Solan area of the Krol Belt, Lesser Himalaya, northern India (Fig. 1). The Krol A Formation was chosen as a target of this study for several reasons. First, previous investigations have shown that the Krol A and the underlying Infra-Krol formations contain microfossils whose preservation mode is similar to those in the Doushantuo Formation in the Yangtze Gorges area. Earlier studies revealed silicified filamentous and coccoidal microfossils from chert nodules in the Infra-Krol Formation of the Nainital area (Acharyya et al., Reference Acharyya, Raha, Das, Moitra, Shukla and Bansal1989; Venkatachala et al., Reference Venkatachala, Shukla, Bansal and Acharyya1990) and the Krol A Formation of the Solan area (Kumar and Rai, Reference Kumar and Rai1992). Subsequent investigations recovered various silicified acanthomorphs and multicellular algae from the Infra-Krol Formation in both the Solan and Nainital areas (Tiwari and Azmi, Reference Tiwari and Azmi1992; Tiwari and Knoll, Reference Tiwari and Knoll1994; Tiwari and Pant, Reference Tiwari and Pant2004; Shukla et al., Reference Shukla, Babu, Mathur and Srivastava2005b; Joshi and Tiwari, Reference Joshi and Tiwari2016), as well as the Krol A Formation in the Solan area (Shukla et al., Reference Shukla, Mathur, Babu and Srivastava2008; Shukla and Tiwari, Reference Shukla and Tiwari2014; Sharma et al., Reference Sharma, Shukla and Sergeev2021) (Table 1). In particular, the report of Tianzhushania spinosa Yin and Li, Reference Yin and Li1978, and T. polysiphonia Yin in Yin and Liu, Reference Yin, Liu, Zhao, Xing, Ding, Liu, Zhao, Zhang, Meng, Yin, Ning and Han1988, from the Infra-Krol Formation on the Nainital area (Joshi and Tiwari, Reference Joshi and Tiwari2016) bolsters a direct biostratigraphic correlation with the lower Doushantuo Formation in the Yangtze Gorges area, where these two taxa are characteristically abundant (McFadden et al., Reference McFadden, Xiao, Zhou and Kowalewski2009; Yin et al., Reference Yin, Liu, Chen, Tang, Gao and Wang2009). Second, the correlation between Ediacaran successions in South China and northern India is facilitated by their paleogeographic proximity during the Ediacaran Period (Jiang et al., Reference Jiang, Sohl and Christie-Blick2003a; Merdith et al., Reference Merdith, Williams, Collins, Tetley, Mulder, Blades, Young, Armistead, Cannon, Zahirovic and Müller2021). Finally, the Krol A Formation consists of interbedded shale and dolostone with fossiliferous chert nodules, offering an opportunity for integrative investigation of acanthomorph biostratigraphy and δ13C chemostratigraphy, given that previous studies of Krol A acanthomorphs (see references above) were decoupled from sequence stratigraphic and δ13C chemostratigraphic investigations (Jiang et al., Reference Jiang, Christie-Blick, Kaufman, Banerjee and Rai2002, Reference Jiang, Christie-Blick, Kaufman, Banerjee and Rai2003b; Kaufman et al., Reference Kaufman, Jiang, Christie-Blick, Banerjee and Rai2006). Thus, the Krol A Formation is an ideal test ground for the bio- and chemostratigraphic framework derived from the Doushantuo Formation South China, particularly the Yangtze Gorges area, because of the lithostratigraphic similarity, paleogeographic proximity, and taphonomic comparability between these two successions. No other Ediacaran succession, to our knowledge, offers such a great opportunity. To take full advantage of this opportunity, we carried out a systematic and integrative paleontological and geochemical analysis of the Krol A Formation in the Solan area.

Figure 1. Simplified geological map showing the exposure of late Neoproterozoic strata (Blaini, Krol, and Tal groups) along the Krol Belt of the Lesser Himalaya, northern India. Modified from Singh and Rai (Reference Singh and Rai1983). Inset map shows location of the Krol Belt in northern India. The geology of the Krol and Pachmunda synclines in the Solan area is provided in Figure 2.
Table 1. Summary of previous reports of acanthomorphic acritarchs from the Infra-Krol and Krol A formations in Lesser Himalaya.

Geological setting
Neoproterozoic strata of the Krol Belt, Lesser Himalaya, northern India crop out in a series of doubly plunging synclines from Solan in the northwest to Nainital in the southeast (Fig. 1) (Auden, Reference Auden1934; Singh and Rai, Reference Singh and Rai1983; Shanker et al., Reference Shanker, Kumar, Mathur and Johsi1993). Following the stratigraphic scheme of Jain et al. (Reference Jain, Banerjee and Kale2020), these strata consist of three parts: (1) Tonian siliciclastic-dominated rocks of the Jaunsar/Simla groups; (2) Cryogenian diamictite, siltstone, and sandstone of the Blaini Group; and (3) Ediacaran shale/siltstone and carbonates of the Krol Group, which includes the Infra-Krol Formation (Jain et al., Reference Jain, Banerjee and Kale2020). There are no precise radioisotopic dates from syndepositional ash beds to constrain the depositional age of these units, but detrital zircon ages indicate that the Jaunsar/Simla groups are likely of Tonian age (≤850 Ma; Frank et al., Reference Frank, Bhargava, Miller and Banerjee2001; McKenzie et al., Reference McKenzie, Hughes, Myrow, Xiao and Sharma2011; Webb et al., Reference Webb, Yin, Harrison, Célérier, Gehrels, Manning and Grove2011), and the glaciogenic rocks of the Blaini Group are of Cryogenian age (≤ 692 ± 18 Ma, Etienne et al., Reference Etienne, Allen, Guerroue, Heaman, Ghosh, Islam, Arnaud, Halverson and Shields-Zhou2011; ≤ 678 ± 10 Ma, Hofmann et al., Reference Hofmann, Linnemann, Rai, Becker, Gärtner and Sagawe2011). The Ediacaran age of the Krol Group is inferred from the occurrence at the top of the Blaini Group of a thin (<10 m) carbonate unit characteristic of the basal Ediacaran cap dolostone (Jiang et al., Reference Jiang, Christie-Blick, Kaufman, Banerjee and Rai2002; Etienne et al., Reference Etienne, Allen, Guerroue, Heaman, Ghosh, Islam, Arnaud, Halverson and Shields-Zhou2011), sequence and δ13C chemostratigraphic correlation with other Ediacaran successions—particularly the Doushantuo and Dengying formations in South China (Jiang et al., Reference Jiang, Christie-Blick, Kaufman, Banerjee and Rai2002, Reference Jiang, Sohl and Christie-Blick2003a; Kaufman et al., Reference Kaufman, Jiang, Christie-Blick, Banerjee and Rai2006), the presence of Ediacaran microfossils in the Infra-Krol and Krol A formations (e.g., Tiwari and Knoll, Reference Tiwari and Knoll1994; Tiwari and Pant, Reference Tiwari and Pant2004; Shukla et al., Reference Shukla, Mathur, Babu and Srivastava2008; Shukla and Tiwari, Reference Shukla and Tiwari2014; Joshi and Tiwari, Reference Joshi and Tiwari2016; Sharma et al., Reference Sharma, Shukla and Sergeev2021), the presence in the overlying Tal Group of early Cambrian acritarchs (Tiwari, Reference Tiwari1999), small shelly fossils (Bhatt et al., Reference Bhatt, Mamgain and Misra1985; Bhatt, Reference Bhatt1991), and trilobites (Hughes et al., Reference Hughes, Peng, Bhargava, Ahluwalia, Walia, Myrow and Parcha2005), as well as the report of the terminal Ediacaran fossil Shaanxilithes ningqiangensis Xing, Yue, and Zhang in Xing et al., Reference Xing, Ding, Luo, He and Wang1984, from the uppermost Krol and basalmost Tal groups (Tarhan et al., Reference Tarhan, Hughes, Myrow, Bhargava, Ahluwalia and Kudryavtsev2014; Bhargava et al., Reference Bhargava, Singh, Frank and Tangri2021).
Ediacaran strata in the Krol Belt were traditionally mapped as Infra-Krol, Krol Sandstone, Krol A, B, C, D, and E units (Figs. 2, 3.1) (Auden, Reference Auden1934; Bhattacharya and Niyogi, Reference Bhattacharya and Niyogi1971). Shanker et al. (Reference Shanker, Kumar, Mathur and Johsi1993, Reference Shanker, Mathur and Kumar1997) recommended raising the Krol to group status and formalized the internal subdivisions of the Krol Group as the Chambaghat Formation (Krol Sandstone), Mahi Formation (Krol A), Jarashi Formation (Krol B), and Kauriyala Formation (Krol C, D, and E). These formation names, however, have not been widely accepted in India. Because the traditional nomenclature (i.e., Krol A, B, C, D, E) has been widely used in geological maps, Jain et al. (Reference Jain, Banerjee and Kale2020) suggested raising the informal letter names to formation status and including the Infra-Krol Formation in the Krol Group (Fig. 3.1). In this paper, we follow the stratigraphic nomenclature of Jain et al. (Reference Jain, Banerjee and Kale2020), who also placed the basal Ediacaran cap carbonate in the uppermost the Blaini Group, although some authors placed it in the basal Infra-Krol Formation (Jiang et al., Reference Jiang, Sohl and Christie-Blick2003a). A particular point that needs to be clarified is the relationship between the Krol Sandstone and Infra-Krol Formation. Because the Krol Sandstone is present only in the Solan and Nainital areas and its immediate overlying strata vary from shale (the definition of the Infra-Krol Formation) to interbedded shaly dolostone and shale (the definition of Krol A Formation), lithostratigraphically the Infra-Krol Formation may extend above the Krol Sandstone in some places (Jiang et al., Reference Jiang, Christie-Blick, Kaufman, Banerjee and Rai2002). With this consideration, the Krol Sandstone may be better defined as a member or an informal lithostratigraphic unit within the Infra-Krol Formation (Fig. 3.1).

Figure 2. Geological map of the Solan area (Krol and Pachmunda synclines) showing the location of measured sections DH-14 and DH2-14. Modified from Auden (Reference Auden1934) and Bhattacharya and Niyogi (Reference Bhattacharya and Niyogi1971).

Figure 3. Litho-, chemo-, and biostratigraphy of the measured sections in the southeastern corner of the Pachmunda syncline (see Fig. 2 for location). (1) Stratigraphic nomenclature of the Ediacaran units in the Krol Belt. (2) Composite stratigraphic log of the measured sections from the topmost Infra-Krol Formation to the Krol C Formation. The stratigraphic position of chert nodule samples is marked, along with carbonate δ13C and δ18O data from Krol A to Krol C. Sample numbers in black contains no acanthomorphs, but are not necessarily non-fossiliferous. (3) δ13C–δ18O cross-plot. The lower–middle Krol A Formation (~40–75 m) has negative δ13C values but consistent δ18O values around −4‰ (brown symbols). The rest of the δ13C and δ18O data are shown in yellow symbols. (4) Stratigraphic occurrence of the leiosphere Schizofusa zangwenlongii, the herkomorph Dictyotidium grazhdankinii Xiao n. sp., and all acanthomorph species recovered from the Krol A Formation. Stratigraphic heights are aligned to the stratigraphic column in (2). Note the occurrence of Appendisphaera grandis, Schizofusa zangwenlongii, and Tanarium cf. T. conoideum. These are either eponymous or morphologically similar species of the three assemblage zones recognized by Liu and Moczydłowska (Reference Liu and Moczydłowska2019) from member II of the Doushantuo Formation in the Yangtze Gorges area (i.e., the Appendisphaera grandis-Weissiella grandistella-Tianzhushania spinosa, the Tanarium tuberosum-Schizofusa zangwenlongii, and the Tanarium conoideum-Cavaspina basiconica assemblage zones). Also note that Liu and Moczydłowska (Reference Liu and Moczydłowska2019) regarded Weissiella brevis, which occurs in the Krol A Formation, as synonymous with W. grandistella.
The measured and sampled sections for this study cover the uppermost Infra-Krol Formation through the lower part of Krol C Formation in the southeastern corner of the Pachmunda syncline in the Solan area (Figs. 2, 3.2). Section DH-14 (N30°53′57.8″, E77°05′14.0″; Fig. 2) was measured through an excavated quarry that covers the top of the Infra-Krol Formation, Krol Sandstone, and Krol A Formation. Section DH2-14 (N30°53′41.3″, E77°05′29.5″; Fig. 2) was measured from the Solan-Barog road towards north along a construction roadcut, and covers the uppermost Krol A, Krol B, and lower Krol C formations.
The Infra-Krol Formation consists of black shales with an up-section increase in siltstone and fine-grained sandstone beds towards the Krol Sandstone. The Krol Sandstone in the measured section (DH-14 in Fig. 2) is ~33 m thick and contains cross stratification in the middle part. At this section, interbedded silty shale and shaly dolostone of the Krol A Formation directly overlie the Krol Sandstone (Fig. 4.1). Black to dark, spherical chert nodules of 0.3–2 cm in diameter (Fig. 4.2, 4.4) and thin (<2 cm), laterally discontinuous chert bands (Fig. 4.3) are found at multiple horizons from the lower to middle Krol A Formation (Fig. 3.2). Towards the upper Krol A Formation (Fig. 4.5), chert nodules become larger in size (up to 7 cm in diameter) and are often flattened along the bedding (Fig. 4.6). The Krol B Formation in the measured section (DH2-14 in Fig. 2) is only 10 m thick and consists of reddish siltstone/mudstone with silty dolostone interbeds. A 0.4-m-thick calcareous sandstone layer marks the top of the Krol B Formation, which is overlain by a 15-m-thick, thinly bedded, calcareous shale and lime mudstone of the lowermost Krol C Formation. The rest of the Krol C Formation consists of black to dark-gray bituminous limestone (Fig. 3.2).

Figure 4. Field photos of the measured sections. (1) Overview of the Krol Sandstone and Krol A Formation in a newly excavated quarry (section DH-14). Outcrop shown here is ~60 m thick (40 m of Krol A and 20 m of Krol Sandstone). (2) Chert nodules in silty dolostone of Krol A (sample DH-14-52.6 in Fig. 3.2). (3) Chert nodules and bands in dolomitic shale and microcrystalline dolostone of Krol A (sample DH-14-64.1 in Fig. 3.2). (4) Chert nodules in silty dolostone of Krol A (sample DH-14-66.0 in Fig. 3.2). (5) Interbedded shale and dolostone of Krol A along the road in section DH2-14 (0.0–3.2 m). (6) Chert nodules in dolomitic shales of Krol A (samples DH2-14-3.1 and S4-4-F1 in Fig. 3.2). There are small (yellow arrows) and large (red arrows) chert nodules in the upper part of Krol A. Large chert nodules typically do not contain fossils. Pencil (14 cm) and pencil head (1.8 cm) for scale in (2–4, 6). Rock hammer (30 cm) for scale in (5) (lower right).
Materials and methods
One hundred eighty rock samples at 0.2–1.0 m stratigraphic spacing were collected from the Krol A–C formations at the study sections for petrographic and geochemical (δ13C and δ18O) analyses. Samples were washed and cut in the laboratory to exposure fresh surfaces for petrographic thin section preparation and geochemical microsampling. Carbonate powders were drilled from fresh surfaces of the samples. For isotope analyses, ~50–200 μg of carbonate powders were allowed to react with orthophosphoric acid for 10 minutes at 70°C, using a Kiel IV carbonate device connected to a Finnigan Delta V Plus mass spectrometer via dual-inlet at the University of Nevada Las Vegas. Isotope values are reported in δ notation relative to Vienna Pee Dee Belemnite standard (VPDB). Analytical uncertainty monitored by NBS-19 and an internal standard was <0.08‰ for both δ13C and δ18O.
Chert nodule samples were collected, along with the geochemical samples, from 13 horizons of the Krol A Formation for micropaleontological study (Fig. 3.2). They were cleaned and embedded in epoxy for the preparation of standard petrographic thin sections. Nodules were not cut with controlled stratigraphic orientations because most were loosened from friable host rock. Thin sections were systematically examined under an Olympus BX-51 and a Zeiss Axioscope A1 transmitted light microscope. Microfossils were positioned using built-in coordinate systems and illustrated microfossils were additionally positioned using an England Finder slide. Selected microfossils were photographed using digital cameras attached to the microscopes. Ninety-four petrographic slides were examined and 274 ornamented acritarch specimens were photographed. The ornamented acritarch taxa are described in Systematic Paleontology because of their biostratigraphic significance. Representative sphaeromorphs, filaments, coccoids, and multicellular algae are illustrated, but not described in detail.
Repositories and institutional abbreviations
All illustrated microfossils are deposited in the Virginia Polytechnic Institute Geosciences Museum (VPIGM). For each illustrated specimen, the thin section number (which contains the sample number, e.g., thin section DH-14-65.0-B comes from sample DH-14-65.0), Olympus BX-51 coordinates (e.g., 14.3 × 134.6), and England Finder coordinates (e.g., EF-Q28-4) are given. Descriptive terminology is adopted from Xiao et al. (Reference Xiao, Zhou, Liu, Wang and Yuan2014). Taxonomic nomenclature follows the International Code of Nomenclature for Algae, Fungi, and Plants (Turland et al., Reference Turland, Wiersema, Barrie, Greuter, Hawksworth, Herendeen, Knapp, Kusber, Li, Marhold, May, McNeill, Monro, Prado, Price and Smith2018).
Systematic paleontology
Group Acritarcha Evitt, Reference Evitt1963
Genus Appendisphaera Moczydłowska, Vidal, and Rudavskaya, Reference Moczydłowska, Vidal and Rudavskaya1993, emend. Moczydłowska, Reference Moczydłowska2005
Type species
Appendisphaera grandis Moczydłowska, Vidal, and Rudavskaya, Reference Moczydłowska, Vidal and Rudavskaya1993, emend. Moczydłowska, Reference Moczydłowska2005.
Other species
Appendisphaera anguina Grey, Reference Grey2005; A.? brevispina Liu et al., Reference Liu, Xiao, Yin, Chen, Zhou and Li2014; A. clava Liu et al., Reference Liu, Xiao, Yin, Chen, Zhou and Li2014; A. clustera Liu and Moczydłowska, Reference Liu and Moczydłowska2019; A. fragilis Moczydłowska, Vidal, and Rudavskaya, Reference Moczydłowska, Vidal and Rudavskaya1993; A. heliaca (Liu and Moczydłowska, Reference Liu and Moczydłowska2019) Ouyang et al., Reference Ouyang, Zhou, Xiao, Guan, Chen, Yuan and Sun2021; A.? hemisphaerica Liu et al., Reference Liu, Xiao, Yin, Chen, Zhou and Li2014; A. lemniscata Liu and Moczydłowska, Reference Liu and Moczydłowska2019; A. longispina Liu et al., Reference Liu, Xiao, Yin, Chen, Zhou and Li2014; A. longitubularis (Liu et al., Reference Liu, Xiao, Yin, Chen, Zhou and Li2014) Liu and Moczydłowska, Reference Liu and Moczydłowska2019, an orthographic correction of A. longitubulare as published in Liu and Moczydłowska (Reference Liu and Moczydłowska2019); A. magnifica (Zhang et al., Reference Zhang, Yin, Xiao and Knoll1998) Liu et al., Reference Liu, Xiao, Yin, Chen, Zhou and Li2014; A. setosa Liu et al., Reference Liu, Xiao, Yin, Chen, Zhou and Li2014; A. tabifica Moczydłowska, Vidal, and Rudavskaya, Reference Moczydłowska, Vidal and Rudavskaya1993; A. tenuis Moczydłowska, Vidal, and Rudavskaya, Reference Moczydłowska, Vidal and Rudavskaya1993.
Remarks
Several Appendisphaera species published in the literature have been synonymized with existing species or transferred to other genera, hence they are not listed above. Liu and Moczydłowska (Reference Liu and Moczydłowska2019, p. 61) considered Appendisphaera barbata Grey, Reference Grey2005, A. centoreticulata Grey, Reference Grey2005, A. dilutopila (Zang in Zang and Walter, Reference Zang and Walter1992) Grey, Reference Grey2005, and A. minutiforma Grey, Reference Grey2005, as junior synonyms of A. tabifica. They also regarded A. minima Nagovitsin and Faizullin in Nagovitsin et al., Reference Nagovitsin, Faizullin and Yakshin2004, as a junior synonym of A. tenuis, and excluded A. crebra (Zang in Zang and Walter, Reference Zang and Walter1992) Liu et al., Reference Liu, Xiao, Yin, Chen, Zhou and Li2014, from the genus Appendisphaera. Liu and Moczydłowska (Reference Liu and Moczydłowska2019) indicated that A. magnifica is synonymous with A. grandis, but did not provide any justification; in this paper we follow Liu et al. (Reference Liu, Xiao, Yin, Chen, Zhou and Li2014a) and Ouyang et al. (Reference Ouyang, Zhou, Xiao, Guan, Chen, Yuan and Sun2021) and regard A. magnifica as a distinct species of Appendisphaera (see discussion under the species A. grandis).
A Doushantuo specimen illustrated in Liu et al. (Reference Liu, Qi, Fan, Guo, Pei, Huang, Cheng, Bian, Liu, Zhao and Zhang2021) as Ericiasphaera magna seems to have hollow rather than solid process (see Liu et al., Reference Liu, Qi, Fan, Guo, Pei, Huang, Cheng, Bian, Liu, Zhao and Zhang2021, fig. 4.5, 4.6), and thus may belong to the genus Appendisphaera. It is somewhat similar to A. setosa or A. tenuis in process density and morphology, particularly the extremely thin processes (~1.0–1.5 μm wide at the base and ~0.3 μm wide above the base).
Appendisphaera clava Liu et al., Reference Liu, Xiao, Yin, Chen, Zhou and Li2014
Figures 5, 6
- Reference Liu, Yin, Chen, Tang and Gao2013
Unnamed (E); Liu et al., fig. 12A, B.
- Reference Liu, Xiao, Yin, Chen, Zhou and Li2014a
Appendisphaera clava Liu et al., p. 12, figs. 5.4, 8.1–8.5, 9.1–9.7.
- Reference Muscente, Hawkins and Xiao2015
Appendisphaera clava; Muscente et al., fig. 5D.
- Reference Ouyang, Zhou, Xiao, Chen and Shao2019
Appendisphaera clava; Ouyang et al., fig. 8G, H (part).
- Reference Grazhdankin, Nagovitsin, Golubkova, Karlova, Kochnev, Rogov and Marusin2020
Appendisphaera clava; Grazhdankin et al., fig. 3C.
- Reference Ouyang, Zhou, Xiao, Guan, Chen, Yuan and Sun2021
Appendisphaera clava; Ouyang et al., fig. 10K, L.

Figure 5. Appendisphaera clava. (1–3) DH-14-67.0-B-2, 20.8 × 111.6, EF-H11-2, VPIGM-4847, rectangle in (1) marks area shown in (2) at a different focal level, arrow in (2) marks area shown in (3) at a different focal level; (4–6) S4-4-F2-7, 3.0 × 139.5, EF-AA39-1, VPIGM-4889, rectangle in (4) marks area shown in (5) at a different focal level and with a slight rotation, arrow in (4) marks area shown in (6) at a different focal level and with a slight rotation; (7, 8) S4-4-F2-5, 23.0 × 107.0, EF-E7-1, VPIGM-4878, arrow in (7) marks area shown in (8) at a different focal level and with a slight rotation. All specimens illustrated in this paper are from the Krol A Formation, Solan, northern India. For each illustrated specimen, the following information is given: thin section number (which is the sample number with a differentiating suffix if multiple thin sections were made from the sample), Olympus BX-51 coordinates, England Finder coordinates, and VPIGM catalog number.

Figure 6. Appendisphaera clava. (1–3) S4-4-F1-4, 17.0 × 124.5, EF-L24-4, VPIGM-4871, rectangle in (1) marks area shown in (2) and (3) at different focal levels; (4, 5) DH-14-67.0-C-2, 13.6 × 134.3, EF-P34-1, VPIGM-4853, rectangle in (4) marks area shown in (5); (6–8) S4-4-F2-7, 6.8 × 139.3, EF-V39-3, VPIGM-4890, rectangle in (6) marks area shown in (7) and (8) at different focal levels.
Holotype
IGCAGS–WFG–676, reposited at Institute of Geology, Chinese Academy of Geological Sciences, from the lower member III of the Ediacaran Doushantuo Formation at Wangfenggang section in the Yangtze Gorges area, Hubei Province, South China (Liu et al., Reference Liu, Xiao, Yin, Chen, Zhou and Li2014a, fig. 8.1, 8.2).
Occurrence
Ediacaran of South China and northern India, and lower Cambrian of Siberia. South China: member II and equivalent strata of the Doushantuo Formation at Jinguadun (Ouyang et al., Reference Ouyang, Zhou, Xiao, Guan, Chen, Yuan and Sun2021) and Wuzhishan (Ouyang et al., Reference Ouyang, Zhou, Xiao, Guan, Chen, Yuan and Sun2021) in the Yangtze Gorges and surrounding areas; member III of the Doushantuo Formation at Wangfenggang and Niuping in the Yangtze Gorges area (Liu et al., Reference Liu, Xiao, Yin, Chen, Zhou and Li2014a). Northern India: Ediacaran Krol A Formation in the Solan area of northern India (this paper). Siberia: upper Ediacaran or lower Cambrian Oppokun Formation, Khastakhskaya borehole, Lena-Anabar Basin, north-central Siberia (Grazhdankin et al., Reference Grazhdankin, Nagovitsin, Golubkova, Karlova, Kochnev, Rogov and Marusin2020).
Description and measurements
Medium-sized to large spherical vesicles with evenly spaced processes that are short, hollow, slightly expanded at base, basally separate, distally pointed, and open to vesicle interior. Vesicle diameter difficult to measure with precision, but likely >200 μm (see Figs. 5.1, 6.1). Approximately 19–34 processes per 100 μm of vesicle periphery, process spacing 1–3 μm at base, process width 2–3 μm at base, and process length 4–11 μm. Basal expansions conical in shape and 1–2 μm in height. Apical spines of processes 2–10 μm in length and ~0.5 μm in maximum width.
Remarks
The Krol A specimens are similar to the holotype of Appendisphaera clava in vesicle size, process density, process morphology, and the size and shape of the basal expansion. The specimens are somewhat similar to A. tenuis in process length and density, but they better conform to the diagnosis of A. clava in its larger vesicle and processes with a more notable basal expansion. For comparison, the holotype of A. clava is 420 μm in vesicle diameter (vs. 87–147 μm in specimens identified as A. tenuis), and its processes have a visible basal expansion and are 12 μm in length (vs. 7–16 μm in A. tenuis) and ~1 μm in process basal width (measurements not reported for A. tenuis); as a result, process length is only 2.9% of vesicle diameter in A. clava (vs. 8–11% in A. tenuis) (Moczydłowska, Reference Moczydłowska2005; Liu et al., Reference Liu, Xiao, Yin, Chen, Zhou and Li2014a).
Ouyang et al. (Reference Ouyang, Zhou, Xiao, Chen and Shao2019) illustrated two specimens of Appendisphaera clava, but one of them (their fig. 8E, F) seems to have long processes (>20 μm in length) and may belong to A. grandis.
Appendisphaera grandis Moczydłowska, Vidal, and Rudavskaya, Reference Moczydłowska, Vidal and Rudavskaya1993, emend. Moczydłowska, Reference Moczydłowska2005
Figure 7
- Reference Moczydłowska, Vidal and Rudavskaya1993
Appendisphaera grandis Moczydłowska et al., p. 503, text-fig. 5, pl. 1, figs. 1, 2.
- Reference Moczydłowska2005
Appendisphaera grandis; Moczydłowska, p. 294, figs. 3, 4.
- non Reference Shukla and Tiwari2014
Appendisphaera grandis; Shukla and Tiwari, p. 215, fig. 4D, E.
- Reference Prasad and Asher2016
Appendisphaera grandis; Prasad and Asher, p. 42, pl. 2, figs. 3, 4.
- non Reference Sharma, Tiwari, Ahmad, Shukla, Shukla, Singh, Pandey, Ansari, Shukla and Kumar2016
Appendisphaera grandis; Sharma et al., fig. 4B.
- Reference Ouyang, Guan, Zhou and Xiao2017
Appendisphaera fragilis Moczydłowska, Vidal, and Rudavskaya; Ouyang et al., fig. 8D–F.
- Reference Anderson, McMahon, Macdonald, Jones and Briggs2019
Appendisphaera grandis; Anderson et al., p. 507, fig. 6A–D.
- Reference Liu and Moczydłowska2019
Appendisphaera grandis; Liu and Moczydłowska, p. 48, figs. 21–23, and synonyms therein (except Appendisphaera? hemisphaerica illustrated in Hawkins et al., Reference Hawkins, Xiao, Jiang, Wang and Shi2017, fig. 9C, D; Meghystrichosphaeridium magnificum illustrated in Zhang et al., Reference Zhang, Yin, Xiao and Knoll1998, fig. 10.5, 10.6; and Liu et al., Reference Liu, Yin, Chen, Tang and Gao2013, fig. 11I, J; and Appendisphaera magnifica illustrated in Liu et al., Reference Liu, Xiao, Yin, Chen, Zhou and Li2014a, figs. 19, 20; and in Hawkins et al., Reference Hawkins, Xiao, Jiang, Wang and Shi2017, fig. 9A, B).
- Reference Shang, Liu and Moczydłowska2019
Appendisphaera grandis; Shang et al., p. 7, fig. 3, and synonyms therein (except Appendisphaera? hemisphaerica illustrated in fig. 9C, D of Hawkins et al., Reference Hawkins, Xiao, Jiang, Wang and Shi2017).
- Reference Ouyang, Zhou, Xiao, Chen and Shao2019
Appendisphaera grandis; Ouyang et al., fig. 8I–K.
- Reference Shang and Liu2020
Appendisphaera grandis; Shang and Liu, p. 156, fig. 4.
- Reference Ouyang, Zhou, Xiao, Guan, Chen, Yuan and Sun2021
Appendisphaera grandis; Ouyang et al., fig. 10M–P.
- Reference Liu, Qi, Fan, Guo, Pei, Huang, Cheng, Bian, Liu, Zhao and Zhang2021
Appendisphaera grandis; Liu et al., fig. 5.4.

Figure 7. Appendisphaera grandis. (1–4) S4-4-F2-5, 10.5 × 132.3, EF-S32-2, VPIGM-4873, rectangle in (1) marks area shown in (2), white and black arrows in (2) mark areas shown in (3) (different focal level) and (4), respectively; (5–8) DH-14-66.0-B-2, 9.8 × 120.8, EF-S21-1, VPIGM-4840, white arrow, black arrow, and rectangle in (5) mark areas shown in (6–8), respectively.
Holotype
PMU-Sib.1-R/63/2, reposited at Uppsala University, from the Ediacaran Khamaka Formation, Zapad 742 borehole at a depth of 1887.0–1894.0 m, Nepa-Botuoba region, Yakutia, Siberian (Moczydłowska, Vidal, and Rudavskaya, Reference Moczydłowska, Vidal and Rudavskaya1993, p. 503, text-fig. 5A–D).
Occurrence
Ediacaran of South China, Siberia, Australia (see Liu and Moczydłowska, Reference Liu and Moczydłowska2019, and Shang et al., Reference Shang, Liu and Moczydłowska2019, for detailed occurrence information), and India (this paper). This species also has been reported from the upper Khesen Formation at Urandush Uul in northern Mongolia (Anderson et al., Reference Anderson, Macdonald, Jones, McMahon and Briggs2017, Reference Anderson, McMahon, Macdonald, Jones and Briggs2019), which is considered terminal Ediacaran in age, although the uppermost Khesen Formation contains Cambrian-age detrital zircons (Anttila and Macdonald, Reference Anttila and Macdonald2020). The occurrence of Appendisphaera grandis in the Semri Group of the Lower Vindhyan Supergroup in the Chambal Valley of eastern Rajasthan of central-western India (Prasad and Asher, Reference Prasad and Asher2016) is intriguing because the Semri Group in central India is widely regarded as Paleo-/Mesoproterozoic in age, ca. 1600 Ma (Rasmussen et al., Reference Rasmussen, Bose, Sarkar, Banerjee, Fletcher and McNaughton2002; Ray et al., Reference Ray, Martin, Veizer and Bowring2002); this record and its age warrants further confirmation because of its profound biostratigraphic implications (Hughes, Reference Hughes2017) and because Appendisphaera grandis is the eponymous species of the early Ediacaran Appendisphaera grandis-Weissiella grandistella-Tianzhushania spinosa Assemblage Zone of Liu and Moczydłowska (Reference Liu and Moczydłowska2019).
Description and measurements
Medium-sized to large spherical vesicles with closely and evenly spaced processes that are long, hollow, cylindrical or slightly expanded at base, distally tapering, and open to vesicle interior. Vesicle diameter difficult to measure with precision due to deformation, but one specimen is ~440 μm in diameter (Fig. 7.5). Approximately 15–50 processes per 100 μm of vesicle periphery, process spacing up to 1.4 μm at base, although many processes are in basal contact with each other, process length 17–21 μm. Most processes are cylindrical (~0.5 μm in width; Fig. 7.6), although some appear to have a basal expansion supporting an apical spine (Fig. 7.3, 7.4, 7.7). We cannot exclude the possibility that the basal expansion is a diagenetic artifact; nonetheless, the apparent basal expansion measures up to 3–4 μm in width and 3–4 μm in height, and the apical spine is 12–17 μm in length and ~0.5 μm in maximum width.
Materials
Two illustrated specimens (Fig. 7) and 18 additional specimens.
Remarks
The Krol A specimens are identified as Appendisphaera grandis based on their relatively long and densely distributed processes. Some, but not all, processes in the Krol A specimens have a slightly expanded base (e.g., Fig. 7.3, 7.4), but they are otherwise similar to the holotype (Moczydłowska et al., Reference Moczydłowska, Vidal and Rudavskaya1993) and other specimens identified as Appendisphaera grandis (Liu and Moczydłowska, Reference Liu and Moczydłowska2019).
Meghystrichosphaeridium magnificum Zhang et al., Reference Zhang, Yin, Xiao and Knoll1998, is somewhat similar to Appendisphaera grandis in vesicle size, process density, and process morphology. Liu et al. (Reference Liu, Xiao, Yin, Chen, Zhou and Li2014a) acknowledged these similarities, but emphasized that the processes of M. magnificum are more regularly and evenly distributed, and that they taper toward a more sharply pointed distal end than those of A. grandis. Thus, they transferred this species to the genus Appendisphaera, but maintained it as a distinct species, A. magnifica. Subsequently, without providing explanation or justification, Liu and Moczydłowska (Reference Liu and Moczydłowska2019) marked M. magnificum as an invalid species and listed it as a junior synonym of A. grandis. As far as we can tell, M. magnificum is an effectively and validly published species (Zhang et al., Reference Zhang, Yin, Xiao and Knoll1998). Not knowing the basis for the synonymization proposed by Liu and Moczydłowska (Reference Liu and Moczydłowska2019), we follow Liu et al. (Reference Liu, Xiao, Yin, Chen, Zhou and Li2014a), Hawkins et al. (Reference Hawkins, Xiao, Jiang, Wang and Shi2017), and Ouyang et al. (Reference Ouyang, Zhou, Xiao, Guan, Chen, Yuan and Sun2021) and treat A. magnifica and A. grandis as distinct taxa.
Liu and Moczydłowska (Reference Liu and Moczydłowska2019) included specimens identified by Hawkins et al. (Reference Hawkins, Xiao, Jiang, Wang and Shi2017) as Appendisphaera? hemisphaerica and A. crebra (Zang in Zang and Walter, Reference Zang and Walter1992) Liu et al., Reference Liu, Xiao, Yin, Chen, Zhou and Li2014 in the synonym list of A. grandis, but no justification was provided. Similarly, Shang et al. (Reference Shang, Liu and Moczydłowska2019) included the Appendisphaera? hemisphaerica specimen illustrated by Hawkins et al. (Reference Hawkins, Xiao, Jiang, Wang and Shi2017) in the synonym list of A. grandis, again without explanation or justification. We re-examined Hawkins et al.'s (Reference Hawkins, Xiao, Jiang, Wang and Shi2017) specimens under a transmitted light microscope by adjusting the focal level, and were able to confirm that the A.? hemisphaerica specimen in Hawkins et al. (Reference Hawkins, Xiao, Jiang, Wang and Shi2017) has basally separate biform processes with a clearly defined basal expansion (~4 μm in diameter) and a thin apical spine (~1 μm in diameter), features that are compatible with A.? hemisphaerica. Although some processes of A. grandis can have a slightly widened base (Moczydłowska, Reference Moczydłowska2005), they are not biform and typically are narrower in basal width (e.g., 1–2 μm, Shang et al., Reference Shang, Liu and Moczydłowska2019; 1–3 μm, Liu and Moczydłowska, Reference Liu and Moczydłowska2019; 2–3 μm, Liu et al., Reference Liu, Qi, Fan, Guo, Pei, Huang, Cheng, Bian, Liu, Zhao and Zhang2021). Thus, the specimen illustrated in Hawkins et al. (Reference Hawkins, Xiao, Jiang, Wang and Shi2017) better fits the diagnosis of A.? hemisphaerica than that of A. grandis. The A. crebra specimen of Hawkins et al. (Reference Hawkins, Xiao, Jiang, Wang and Shi2017) is poorly preserved, and may be assigned to A. grandis given that the holotype of A. crebra may not belong to the genus Appendisphaera (Liu and Moczydłowska, Reference Liu and Moczydłowska2019).
A specimen illustrated as Appendisphaera fragilis in Ouyang et al. (Reference Ouyang, Guan, Zhou and Xiao2017) has longer and more densely arranged processes than the holotype of A. fragilis, but better fits the diagnosis of A. grandis; this specimen is also listed as a synonym of A. grandis in Liu and Moczydłowska (Reference Liu and Moczydłowska2019), Shang et al. (Reference Shang, Liu and Moczydłowska2019), and Shang and Liu (Reference Shang and Liu2020), but only the latter authors offered an explanation.
Specimens identified as Appendisphaera grandis from the Semri Group of the Lower Vindhyan Supergroup in the Chambal Valley of eastern Rajasthan of India (Prasad and Asher, Reference Prasad and Asher2016) do have thin and densely distributed processes, but their vesicles (50–80 μm in diameter) are smaller than the holotype of A. grandis (105–108 μm in diameter; Moczydłowska et al., Reference Moczydłowska, Vidal and Rudavskaya1993). As mentioned above, the occurrence of A. grandis in the Semri Group needs to be verified, considering its profound biostratigraphic implications (Hughes, Reference Hughes2017).
We agree with Liu and Moczydłowska (Reference Liu and Moczydłowska2019) that the two specimens illustrated as A. grandis in Shukla and Tiwari (Reference Shukla and Tiwari2014), one of which was also illustrated in Sharma et al. (Reference Sharma, Tiwari, Ahmad, Shukla, Shukla, Singh, Pandey, Ansari, Shukla and Kumar2016), are better assigned to A. tenuis, because their processes are proportionally shorter than those in A. grandis.
Appendisphaera? hemisphaerica Liu et al., Reference Liu, Xiao, Yin, Chen, Zhou and Li2014
Figures 8–12
- Reference Liu, Xiao, Yin, Chen, Zhou and Li2014a
Appendisphaera? hemisphaerica Liu et al., p. 17, figs. 13–15.
- non Reference Ouyang, Zhou, Guan and Wang2015
Appendisphaera? hemisphaerica; Ouyang et al., p. 215, pl. I, figs. 3, 5.
- Reference Hawkins, Xiao, Jiang, Wang and Shi2017
Appendisphaera? hemisphaerica; Hawkins et al., fig. 9C, D.
- Reference Shang, Moczydłowska, Liu and Liu2018
Appendisphaera? hemisphaerica; Shang et al., fig. 4B.
- Reference Shang, Liu and Moczydłowska2019
Appendisphaera? hemisphaerica; Shang et al., p. 7, fig. 4A, B.

Figure 8. Appendisphaera? hemisphaerica. (1–3) DH-14-67.0-A-2, 15.8 × 111.7, EF-M11-4, VPIGM-4842, black and white arrows in (1) mark areas shown in (2, 3), respectively, at different focal levels; (4, 5) DH-14-67.0-A-2, 18.8 × 117.9, EF-J17-4, VPIGM-4843, arrow in (4) marks area shown in (5) at a different focal level; (6–8) S4-4-F2-7, 17.0 × 125.9, EF-L26-3, VPIGM-4887, rectangle in (6) marks area shown in (7), arrow in (7) marks area shown in (8) with a 180° rotation; (9, 10) DH-14-66.0-B-2, 11.4 × 107.4, EF-Q7-4, VPIGM-4839, arrow in (9) marks area shown in (10) at a different focal level.
Holotype
IGCAGS–WFG–248, reposited at Institute of Geology, Chinese Academy of Geological Sciences, from the lower member III of the Ediacaran Doushantuo Formation at Wangfenggang section in the Yangtze Gorges area, Hubei Province, South China (Liu et al., Reference Liu, Xiao, Yin, Chen, Zhou and Li2014a, fig. 13.1–13.3).
Occurrence
Ediacaran of South China and northern India. South China: member II of the Doushantuo Formation at Siduping section in the Zhangjiajie area, Hunan Province (Hawkins et al., Reference Hawkins, Xiao, Jiang, Wang and Shi2017); member III of the Doushantuo Formation at Wangfenggang and Niuping sections in the Yangtze Gorges area of Hubei Province (Liu et al., Reference Liu, Xiao, Yin, Chen, Zhou and Li2014a); Doushantuo Formation at Liujing section in Guizhou Province (Shang et al., Reference Shang, Liu and Moczydłowska2019). Northern India: Krol A Formation at Solan of northern India (this paper).
Description and measurements
Medium-sized to large spherical vesicles with closely and evenly spaced biform processes that are characterized by an easily recognizable basal expansion subtending a thin and long apical spine. Processes open to vesicle interior. Vesicle diameter ~300 μm, as estimated from three specimens (Figs. 8.1, 8.9, 9.3). Approximately 13–21 processes per 100 μm of vesicle periphery, process spacing 1–3 μm at base, but many processes are in basal contact, and process length 12–29 μm. Basal expansion conical and often inflated (Fig. 9.2, 9.5, 9.7), 3–6 μm in width, and 2–4 μm in height. Apical spine thin and cylindrical in shape, ~1 μm in width, and 7–25 μm in length.

Figure 9. Appendisphaera? hemisphaerica. (1, 2) DH-14-67.0-A-2, 24.0 × 117.8, EF-D17-2, VPIGM-4844, arrow in (1) marks area shown in (2) at a different focal level; (3–5) S4-4-F2-5-2, 2.2 × 130.5, EF-AA31-1, VPIGM-4883, rectangle and arrow in (3) mark areas shown in (4, 5), respectively, at different focal levels; (6, 7) S4-4-F2-5-2, 5.6×129.0, EF-W29-4, VPIGM-4884, arrow in (6) marks area shown in (7) at a different focal level; (8–10) S4-4-F1-3, 16.3 × 125.9, EF-M26-1, VPIGM-4870, rectangle in (8) marks area shown in (9, 10) at different focal levels.
Materials
Eighteen illustrated specimens (Figs. 8–12) and six additional specimens.

Figure 10. Appendisphaera? hemisphaerica. (1–4) S4-4-F2-15, 13.0 × 139.0, EF-P39-1, VPIGM-4899, rectangle in (1) marks area shown in (2), arrow in (1) marks area shown in (3, 4) at different focal levels and with slight rotations; (5–8) S4-4-F2-7, 10.3 × 129.3, EF-S29, VPIGM-4885, rectangles in (5, 6) mark areas shown in (6, 7), respectively, and arrow in (5) marks area shown in (8) at a different focal level and with a slight rotation; (9, 10) S4-4-F2-15, 21.9 × 138.0, EF-F38-3, VPIGM-4901, rectangle in (9) marks area shown in (10).

Figure 11. Appendisphaera? hemisphaerica. (1–3) DH-14-67.0-C-2, 11.1 × 140.8, EF-Q41-3, VPIGM-4851, (1) and (2) show roughly the same area at different focal levels, rectangle in (2) marks area shown in (3); (4–6) S4-4-F2-5-2, 18.5 × 140.5, EF-K40-2, VPIGM-4882, white and black arrows in (4) mark areas shown in (5, 6), respectively, at different focal levels; (7–10) DH-14-67.0-C-2, 15.8 × 141.4, EF-M41-3/4, VPIGM-4856, rectangle in (7) marks area shown in (8), white and black arrows in (8) mark areas shown in (9) (at a different focal level) and (10), respectively.

Figure 12. Appendisphaera? hemisphaerica. (1, 2) DH-14-67.0-A-2, 24.3 × 112.3, EF-D12, VPIGM-4845, rectangle in (1) marks area shown in (2) at a different focal level; (3–5) DH-14-68.0-B-2, 10.0 × 106.3, EF-T7-1, VPIGM-4865, rectangle and arrow in (3) mark areas shown in (4) and (5) (at a different focal level), respectively; (6, 7) S4-4-F2-5, 11.8 × 114.3, EF-Q14-4, VPIGM-4874, rectangle in (6) marks area shown in (7) at a different focal level; (8, 9) S4-4-F2-7, 13.2 × 108.5, EF-P8, VPIGM-4886, rectangle in (8) marks area shown in (9).
Remarks
Appendisphaera? hemisphaerica has a combination of features that are characteristic of Appendisphaera (thin and densely distributed processes) and Mengeosphaera (biform processes with a prominent basal expansion). For this reason, this species was tentatively placed in the genus Appendisphaera (Liu et al., Reference Liu, Xiao, Yin, Chen, Zhou and Li2014a). Appendisphaera? hemisphaerica is similar to several Mengeosphaera species in biform processes with a relatively long apical spine, such as M. gracilis Liu et al., Reference Liu, Xiao, Yin, Chen, Zhou and Li2014, M. latibasis Liu et al., Reference Liu, Xiao, Yin, Chen, Zhou and Li2014, and M. uniformis Liu et al., Reference Liu, Xiao, Yin, Chen, Zhou and Li2014. The main differentiator is the size and shape of the basal expansion. For reference, the basal expansion is 7–8 μm, 10–15 μm, and ~5 μm wide, respectively, for the holotypes of the three Mengeosphaera species listed above. Both M. latibasis and M. uniformis have an obtusely domical basal expansion, whereas M. gracilis has a conical basal expansion. However, specimens illustrated as Mengeosphaera gracilis in Liu and Moczydłowska (Reference Liu and Moczydłowska2019) have measurements of process size, shape, and density overlapping those of the holotype of A.? hemisphaerica. It is possible that A.? hemisphaerica and Mengeosphaera gracilis are synonymous, in which case the former species would take priority. At present, we follow Liu et al. (Reference Liu, Xiao, Yin, Chen, Zhou and Li2014a) and treat A.? hemisphaerica and Mengeosphaera gracilis as two distinct species, with the processes of the latter species bearing a relatively larger basal expansion and a relatively shorter apical spine.
A specimen illustrated as Appendisphaera? hemisphaerica in Ouyang et al. (Reference Ouyang, Zhou, Guan and Wang2015) was subsequently identified by Ouyang et al. (Reference Ouyang, Zhou, Xiao, Guan, Chen, Yuan and Sun2021) as Appendisphaera heliaca (Liu and Moczydłowska, Reference Liu and Moczydłowska2019) Ouyang et al., Reference Ouyang, Zhou, Xiao, Guan, Chen, Yuan and Sun2021, because the basal expansions of the processes in this specimen are thought to be a taphonomic artifact related to degradation. As discussed under Appendisphaera grandis, the specimen illustrated as A.? hemisphaerica in Hawkins et al. (Reference Hawkins, Xiao, Jiang, Wang and Shi2017) has basally separate biform processes with a clearly defined basal expansion. Thus, this specimen belongs to A.? hemisphaerica rather than A. grandis.
Appendisphaera longispina Liu et al., Reference Liu, Xiao, Yin, Chen, Zhou and Li2014
Figures 13, 14
- Reference Liu, Xiao, Yin, Chen, Zhou and Li2014a
Appendisphaera longispina Liu et al., p. 21, figs. 17, 18, and synonyms therein.
- Reference Liu, Xiao, Yin, Chen, Zhou and Li2014a
Appendisphaera crebra (Zang in Zang and Walter, Reference Zang and Walter1992); Liu et al., p. 17, figs. 10, 11.
- Reference Liu and Moczydłowska2019
Appendisphaera longispina; Liu and Moczydłowska, p. 54, fig. 25.
- Reference Shang, Liu and Moczydłowska2019
Appendisphaera longispina; Shang et al., p. 8, fig. 4C, D.
- Reference Ouyang, Zhou, Xiao, Guan, Chen, Yuan and Sun2021
Appendisphaera longispina; Ouyang et al., fig. 11G, H.

Figure 13. Appendisphaera longispina. (1–3) DH-14-67.0-C, 8.7 × 127.2, EF-T27, VPIGM-4850, rectangle and arrow in (1) mark areas shown in (2, 3), respectively, at different focal levels; (4–6) DH-14-67.0-C, 14.0 × 133.3, EF-N33-4, VPIGM-4849, white and black arrows in (4) mark areas shown in (5, 6), respectively, at different focal levels; (7, 8) DH-14-67.0-C-2, 18.7 × 133.2, EF-K33-1, VPIGM-4857, arrow in (7) marks area shown in (8).

Figure 14. Appendisphaera longispina. (1, 2) DH-14-68.0-B, 11.5 × 140.1, EF-Q40-1, VPIGM-4864, arrow in (1) marks area shown in (2); (3–5) S4-4-F2-8-A, 17.3 × 109.6, EF-K9-4, VPIGM-4906, rectangle in (3) marks area shown in (4, 5) at two different focal levels; (6–8) S4-4-F2-15, 14.2 × 140.4, EF-O40, VPIGM-4900, rectangle and arrow in (6) mark areas shown in (7, 8), respectively, at different focal levels.
Holotype
IGCAGS–NPIII–141, reposited at Institute of Geology, Chinese Academy of Geological Sciences, from the upper member III of the Ediacaran Doushantuo Formation at Niuping section in the Yangtze Gorges area, Hubei Province, South China (Liu et al., Reference Liu, Xiao, Yin, Chen, Zhou and Li2014a, fig. 18.3, 18.4).
Occurrence
Ediacaran of South China and northern India. South China: member II of the Doushantuo Formation at Jiuqunao and Xiaofenghe sections (Liu and Moczydłowska, Reference Liu and Moczydłowska2019) and at Wuzhishan section (Ouyang et al., Reference Ouyang, Zhou, Xiao, Guan, Chen, Yuan and Sun2021), Yangtze Gorges area, Hubei Province; member III of the Doushantuo Formation at Niuping section in the Yangtze Gorges area, Hubei Province (described as A. crebra and A. longispina in Liu et al., Reference Liu, Xiao, Yin, Chen, Zhou and Li2014a); Doushantuo Formation at Liujing section in Guizhou Province (Shang et al., Reference Shang, Liu and Moczydłowska2019). Northern India: Krol A Formation in the Solan area (this paper).
Description and measurements
Large spherical vesicles with long, homomorphic, and evenly spaced processes that have a conical basal expansion gradually transitioning into a thin apical spine. Processes open to vesicle interior. Vesicle diameter ~250–300 μm, as estimated from two specimens (Fig. 13.1, 13.4). Processes 21–32 μm in length (~10% of vesicle diameter), densely distributed, ~16–24 processes per 100 μm of vesicle periphery, mostly in contact at base, but can be spaced at 1–2 μm. Basal expansion conical or slightly deflated (Fig. 13.2), 3–5 μm in width, and 2–5 μm in height. Apical spine thin and cylindrical, ~1 μm in width, and 19–30 μm in length.
Remarks
Appendisphaera longispina is somewhat similar to A. grandis and A.? hemisphaerica. However, the basal expansion in A. longispina is more prominent than in A. grandis. Relative to A.? hemisphaerica, A. longispina has longer processes, a taller or longer basal expansion, and a more gradual transition from the basal expansion to the apical spine. The current specimens better fit the diagnosis of A. longispina than A.? hemisphaerica.
Following Liu and Moczydłowska (Reference Liu and Moczydłowska2019), specimens illustrated by Liu et al. (Reference Liu, Xiao, Yin, Chen, Zhou and Li2014a) as Appendisphaera crebra (Zang in Zang and Walter, Reference Zang and Walter1992) Liu et al., Reference Liu, Xiao, Yin, Chen, Zhou and Li2014 are transferred to Appendisphaera longispina.
Appendisphaera setosa Liu et al., Reference Liu, Xiao, Yin, Chen, Zhou and Li2014
Figures 15, 16
- Reference Liu, Xiao, Yin, Chen, Zhou and Li2014a
Appendisphaera setosa Liu et al., p.31, figs. 21, 22, and synonyms therein.
- Reference Liu and Moczydłowska2019
Appendisphaera setosa; Liu and Moczydłowska, p. 56, fig. 27.
- Reference Shang, Liu and Moczydłowska2019
Appendisphaera setosa; Shang et al., p. 10, fig. 4E–J.
- ?Reference Grazhdankin, Nagovitsin, Golubkova, Karlova, Kochnev, Rogov and Marusin2020
Appendisphaera setosa; Grazhdankin et al., fig. 3A.
- Reference Ouyang, Zhou, Xiao, Guan, Chen, Yuan and Sun2021
Appendisphaera setosa; Ouyang et al., fig. 11K, O.

Figure 15. Appendisphaera setosa. (1–6) S4-4-F2-5, 19.4 × 131.9, EF-J32-1, VPIGM-4875, rectangle in (1) marks area shown in (2, 3) at different focal levels, white arrow in (3) marks area shown in (4), and white and black arrows in (1) mark areas shown in (5, 6), respectively; (7–9) DH-14-66.0-C-2, 11.9 × 117.3, EF-Q17-2, VPIGM-4841, arrow and rectangle in (7) mark areas shown in (8, 9), respectively, at a different focal level.

Figure 16. Appendisphaera setosa. (1, 2) DH-14-65.0-D, 11.6 × 140.6, EF-Q40-2, VPIGM-4837, rectangle in (1) marks area shown in (2); (3–8) S4-4-F2-18A, 21.5 × 133.2, EF-E33-3, VPIGM-4910, rectangle and white arrow in (3) mark areas shown in (4, 5), respectively; (6–8) show the same area indicated by the black arrow in (3) at different focal levels.
Holotype
IGCAGS–NPIII–592, reposited at Institute of Geology, Chinese Academy of Geological Sciences, from the upper member III of the Ediacaran Doushantuo Formation at Niuping section in the Yangtze Gorges area, Hubei Province, South China (Liu et al., Reference Liu, Xiao, Yin, Chen, Zhou and Li2014a, fig. 22.8, 22.9).
Occurrence
Ediacaran of South China and northern India, and possibly early Cambrian of northern Siberia. South China: member II of the Doushantuo Formation at Jinguadun and Wuzhishan sections, Yangtze Gorges area, Hubei Province (Ouyang et al., Reference Ouyang, Zhou, Xiao, Guan, Chen, Yuan and Sun2021); member III of the Doushantuo Formation at Niuping and Wangfenggang sections (Liu et al., Reference Liu, Xiao, Yin, Chen, Zhou and Li2014a) as well as Baiguoyuan, Dishuiyan, and Chenjiayuanzi sections (Liu and Moczydłowska, Reference Liu and Moczydłowska2019), Yangtze Gorges area, Hubei Province; Doushantuo Formation at Liujing section in Guizhou Province (Shang et al., Reference Shang, Liu and Moczydłowska2019). Northern India: Ediacaran Krol A Formation in the Solan area (this paper). Possible occurrence in Siberia: upper Ediacaran or lower Cambrian Oppokun Formation, Khastakhskaya borehole, Lena-Anabar Basin, north-central Siberia (Grazhdankin et al., Reference Grazhdankin, Nagovitsin, Golubkova, Karlova, Kochnev, Rogov and Marusin2020).
Description and measurements
Specimens assigned to this species are characterized by large vesicles and thin, cylindrical, hollow, homomorphic, evenly distributed, basally separate, and relatively straight processes that lack a basal expansion. Processes open to vesicle interior (Fig. 15.4), but the communication between hollow process and vesicle interior is often obscured by the accumulation of organic matter within the extremely thin processes. Vesicle diameter ~250 μm, as estimated from one completely preserved specimen (Fig. 15.1). Processes 19–29 μm in length (~11% of vesicle diameter, estimated from specimen in Fig. 15.1) and ~1.5 μm in diameter, ~9–12 processes per 100 μm of vesicle periphery, and process spacing 7–18 μm.
Remarks
Appendisphaera setosa is somewhat similar to A. tenuis and A. fragilis. However, A. tenuis has relatively shorter and slightly conical processes. The holotype of A. fragilis is poorly preserved, with a small number of cylindrical processes covering a small area of the vesicle (Moczydłowska et al., Reference Moczydłowska, Vidal and Rudavskaya1993, text-fig. 6A, B). Although its process length (11–20 μm; Moczydłowska et al., Reference Moczydłowska, Vidal and Rudavskaya1993) is comparable to that of the holotype of A. setosa (16 μm; Liu et al., Reference Liu, Xiao, Yin, Chen, Zhou and Li2014a), the proportional process length is much greater in A. fragilis (16–19% of vesicle diameter; Moczydłowska, Reference Moczydłowska2005) than in A. setosa (estimated ~10% of vesicle diameter; Liu et al., Reference Liu, Xiao, Yin, Chen, Zhou and Li2014a). On the other hand, specimens illustrated as A. fragilis in Shang et al. (Reference Shang, Liu and Moczydłowska2019) have much smaller proportional process length (e.g., 7–11% of vesicle diameter) relative to the holotype. Considering their relatively large vesicles and relatively straight processes, which are characteristic of A. setosa, the Krol A specimens are better placed in A. setosa than in A. fragilis.
A specimen illustrated as Appendisphaera setosa (Grazhdankin et al., Reference Grazhdankin, Nagovitsin, Golubkova, Karlova, Kochnev, Rogov and Marusin2020, fig. 3A) is similar to the holotype in vesicle size, process width, and absolute and proportional process length. However, some of its processes have a slightly expanded base. Thus, we regard its identification as A. setosa provisional. A possible alternative would be A. tenuis.
Appendisphaera tenuis Moczydłowska, Vidal, and Rudavskaya, Reference Moczydłowska, Vidal and Rudavskaya1993, emend. Moczydłowska, Reference Moczydłowska2005
Figure 17
- Reference Moczydłowska, Vidal and Rudavskaya1993
Appendisphaera tenuis Moczydłowska et al., p. 506, text-fig. 7.
- Reference Moczydłowska2005
Appendisphaera tenuis; emend. Moczydłowska, p. 296, fig. 5.
- Reference Shukla and Tiwari2014
Appendisphaera grandis; Shukla and Tiwari, p. 215, fig. 4D, E.
- Reference Sharma, Tiwari, Ahmad, Shukla, Shukla, Singh, Pandey, Ansari, Shukla and Kumar2016
Appendisphaera grandis; Sharma et al., fig. 4B.
- Reference Prasad and Asher2016
Appendisphaera tenuis; Prasad and Asher, p. 44, pl. 3, figs. 3–6.
- Reference Liu and Moczydłowska2019
Appendisphaera tenuis; Liu and Moczydłowska, p. 61, figs. 29, 30, and synonyms therein.
- Reference Anderson, McMahon, Macdonald, Jones and Briggs2019
Appendisphaera tenuis; Anderson et al., p. 509, fig. 6H, I.
- Reference Shang, Liu and Moczydłowska2019
Appendisphaera tenuis; Shang et al., p. 10, fig. 5.
- Reference Shang and Liu2020
Appendisphaera tenuis; Shang and Liu, p. 157, fig. 5A, B.
- Reference Vorob'Eva and Petrov2020
Appendisphaera tenuis; Vorob'Eva and Petrov, p. 370, pl. I, figs. 3, 4.
- Reference Ouyang, Zhou, Xiao, Guan, Chen, Yuan and Sun2021
Appendisphaera tenuis; Ouyang et al., fig. 11Q, R.

Figure 17. Appendisphaera tenuis. (1, 2) S4-4-F2-6-A, 9.3 × 110.7, EF-T10-2, VPIGM-4904, arrow in 1 marks area shown in (2); (3, 4) S4-4-F2-12-A, 12.0 × 142.8, EF-Q42-2, VPIGM-4907, arrow in (3) marks area shown in (4); (5–8) DH-14-67.0-C-2, 11.1 × 139.3, EF-Q39-4, VPIGM-4858, white and black rectangles in (5) mark areas magnified in (6, 7), respectively, and (8) illustrates the same area as (7) at a different focal level, showing the hollow nature of processes, as seen in transverse cross section.
Holotype
PMU-Sib.1-M/33, reposited at Uppsala University, from the Ediacaran Khamaka Formation, Zapad 742 borehole at a depth of 1887.0–1894.0 m, Nepa-Botuoba region, Yakutia, Siberian (Moczydłowska et al., Reference Moczydłowska, Vidal and Rudavskaya1993, p. 506, text-fig. 7).
Occurrence
Ediacaran of South China, Siberia, Australia, and India (see Liu and Moczydłowska, Reference Liu and Moczydłowska2019; Shang et al., Reference Shang, Liu and Moczydłowska2019, for detailed occurrence information). Appendisphaera tenuis has been reported from the upper Khesen Formation at Urandush Uul in northern Mongolia (Anderson et al., Reference Anderson, Macdonald, Jones, McMahon and Briggs2017, Reference Anderson, McMahon, Macdonald, Jones and Briggs2019), which is regarded as terminal Ediacaran, although the uppermost Khesen Formation contains Cambrian-age detrital zircons (Anttila and Macdonald, Reference Anttila and Macdonald2020). It has also been reported from the Semri Group of the Lower Vindhyan Supergroup in the Chambal Valley of eastern Rajasthan of central-western India (Prasad and Asher, Reference Prasad and Asher2016). As discussed under Appendisphaera grandis, the Semri Group in central India is widely regarded as Paleo-/Mesoproterozoic in age (Rasmussen et al., Reference Rasmussen, Bose, Sarkar, Banerjee, Fletcher and McNaughton2002; Ray et al., Reference Ray, Martin, Veizer and Bowring2002), and it is important to verify the occurrence of Appendisphaera grandis and A. tenuis in this unit.
Description and measurements
Large vesicles with short, thin, hollow, slightly conical, evenly spaced, and basally separate processes. Vesicle ~265–364 μm in diameter (Fig. 17.1, 17.5; the specimen illustrated in Fig. 17.3 is poorly preserved, but has a medium-sized vesicle). Approximately 22–33 processes per 100 μm of vesicle periphery, process length 7–12 μm (or 2–3% of vesicle diameter), process spacing 2–4 μm at base, and process width 0.7–0.9 μm. Some processes in the specimen illustrated in Fig. 17.5–17.8 appear to have an expanded base (~2 μm wide and ~1.3 μm high), but this is an inconsistent feature (e.g., Fig. 17.6, 17.7) and seems an artifact resulting from degradation of the vesicle wall. Thus, we choose to place this specimen in Appendisphaera tenuis rather than A. clava.
Materials
Three illustrated specimens (Fig. 17) and 33 additional specimens.
Remarks
The Krol A specimens are identified as Appendisphaera tenuis based on their short, thin, hollow, and slightly conical processes, although they are larger in vesicle size than the holotype. Appendisphaera tenuis is similar to A. clava and Cymatiosphaeroides forabilatus in having relatively short processes. However, the processes of A. clava are more densely arranged and have a well-defined, albeit small basal expansion, and C. forabilatus has presumably solid processes that penetrate an outer membrane. Admittedly, when poorly preserved, these features can be difficult to discern. For example, strong degradation and displacement of organic matter by mineral recrystallization at the junction between cell wall and basal processes may give a false impression of a basal expansion, and hollow processes may appear solid due to accumulation of organic matter within the processes. In such cases, we depend on consistent process morphology and coherent preservation of organic walls to make a taxonomic decision, but even so, there are specimens that cannot be confidently assigned to one versus another species.
As discussed under Appendisphaera grandis, the two specimens illustrated as A. grandis in Shukla and Tiwari (Reference Shukla and Tiwari2014) and in Sharma et al. (Reference Sharma, Tiwari, Ahmad, Shukla, Shukla, Singh, Pandey, Ansari, Shukla and Kumar2016) have been re-assigned to A. tenuis because of their short processes (Liu and Moczydłowska, Reference Liu and Moczydłowska2019). Also, as discussed under Appendisphaera setosa, a Cambrian acanthomorph identified as A. setosa (Grazhdankin et al., Reference Grazhdankin, Nagovitsin, Golubkova, Karlova, Kochnev, Rogov and Marusin2020, fig. 3A) may belong to A. tenuis, although a closer examination is needed to confirm or reject this suspicion.
Finally, specimens identified as A. tenuis from the Semri Group of the Lower Vindhyan Supergroup in the Chambal Valley of eastern Rajasthan of India (Prasad and Asher, Reference Prasad and Asher2016) have important biostratigraphic implications if the hosting rocks turn out to be Mesoproterozoic (Hughes, Reference Hughes2017). The Semri specimens have relatively smaller vesicles (50–80 μm in diameter) than the holotype of A. tenuis (115–148 μm in diameter; Moczydłowska et al., Reference Moczydłowska, Vidal and Rudavskaya1993), and as such, their relative process length (as a percentage of vesicle diameter) is greater, but they are otherwise similar to the holotype in process density and absolute process length. Perhaps both A. grandis and A. tenuis have extremely long stratigraphic ranges, from the Paleo–Mesoproterozoic (Prasad and Asher, Reference Prasad and Asher2016) to the terminal Ediacaran–Cambrian (Anderson et al., Reference Anderson, McMahon, Macdonald, Jones and Briggs2019; Grazhdankin et al., Reference Grazhdankin, Nagovitsin, Golubkova, Karlova, Kochnev, Rogov and Marusin2020).
Genus Asterocapsoides Yin and Li, Reference Yin and Li1978, emend. Xiao et al., Reference Xiao, Zhou, Liu, Wang and Yuan2014
Type species
Asterocapsoides sinensis Yin and Li, Reference Yin and Li1978, emend. Xiao et al., Reference Xiao, Zhou, Liu, Wang and Yuan2014.
Other species
Asterocapsoides fluctuensis Liu and Moczydłowska, Reference Liu and Moczydłowska2019; A. robustus Xiao et al., Reference Xiao, Zhou, Liu, Wang and Yuan2014; A. wenganensis (Chen and Liu, Reference Chen and Liu1986) Xiao et al., Reference Xiao, Zhou, Liu, Wang and Yuan2014.
Remarks
In addition to the named species, several unnamed specimens of Asterocapsoides have been reported from Ediacaran deposits, including (1) Asterocapsoides sp. from the Infra-Krol Formation in the Solan area of the Lesser Himalaya, northern India (Tiwari and Knoll, Reference Tiwari and Knoll1994; Tiwari and Pant, Reference Tiwari and Pant2004), which may be A. wenganensis; (2) Asterocapsoides sp. A and sp. B from the Krol A Formation in the Khanog and Rajgarh synclines of the Lesser Himalaya, northern India (Shukla and Tiwari, Reference Shukla and Tiwari2014), which have acutely conical processes (<10 μm in length) that are much shorter than those of existing species of Asterocapsoides; (3) Asterocapsoides sp. from the Doushantuo Formation at Baizhu of Hubei Province, South China (Yang et al., Reference Yang, Pang, Chen, Zhong and Yang2020), which resembles A. wenganensis, but has occasionally branching processes; (4) two specimens of Asterocapsoides sp. from the Doushantuo Formation at Chaoyang of Jiangxi Province, South China (Zhou et al., Reference Zhou, Chen and Xue2002), one of which has been assigned to A. sinensis by Xiao et al. (Reference Xiao, Zhou, Liu, Wang and Yuan2014); and (5) two specimens of Asterocapsoides sp. from the Vychegda Formation at Keltma, Timan Ridge, East European Platform, Russia (Vorob'Eva et al., Reference Vorob'Eva, Sergeev and Knoll2009), one of which may be A. sinensis (see Remarks under A. sinensis).
Asterocapsoides sinensis Yin and Li, Reference Yin and Li1978, emend. Xiao et al., Reference Xiao, Zhou, Liu, Wang and Yuan2014
Figure 18
- Reference Yin and Li1978
Asterocapsoides sinensis Yin and Li, p. 87, pl. 9, fig. 7.
- ?Reference Knoll1992
Asterocapsoides sinensis; Knoll, p. 762, pl. 6, figs. 5, 6.
- Reference Zhang, Yin, Xiao and Knoll1998
Asterocapsoides sinensis; Zhang et al., p. 24, fig. 5.10 (neotype).
- Reference Yuan, Xiao, Yin, Knoll, Zhou and Mu2002
Asterocapsoides sinensis; Yuan et al., p. 70, fig. 87.
- Reference Zhou, Chen and Xue2002
Asterocapsoides sp.; Zhou et al., pl. 2, fig. 6 (part).
- ?Reference Tiwari and Pant2004
Asterocapsoides sinensis; Tiwari and Pant, p. 10, fig. 5C–F.
- Reference Yin, Liu, Gao, Wang, Tang and Liu2007
Asterocapsoides sinensis; Yin et al., pl. 13, fig. 1.
- Reference Liu, Yin, Gao, Tang and Chen2009
Asterocapsoides sinensis; Liu et al., fig. 2g.
- Reference Vorob'Eva, Sergeev and Knoll2009
Asterocapsoides sp.; Vorob'Eva et al., p. 175, fig. 7.10 (part).
- ?Reference Sharma, Kumar, Tiwari, Shukla, Pandey, Srivastava and Banerjee2012
Asterocapsoides sinensis; Sharma et al., fig. 4k, l.
- Reference Xiao, Zhou, Liu, Wang and Yuan2014
Asterocapsoides sinensis; Xiao et al., p. 11, fig. 5.1–5.3, and synonyms therein.
- Reference Liu, Xiao, Yin, Chen, Zhou and Li2014a
Asterocapsoides sinensis; Liu et al., p. 31, fig. 24.1, 24.2.
- Reference Hawkins, Xiao, Jiang, Wang and Shi2017
Asterocapsoides sinensis; Hawkins et al., fig. 8F.
- ?Reference Sharma, Shukla and Sergeev2021
Asterocapsoides sinensis; Sharma et al., fig. 9B, C.
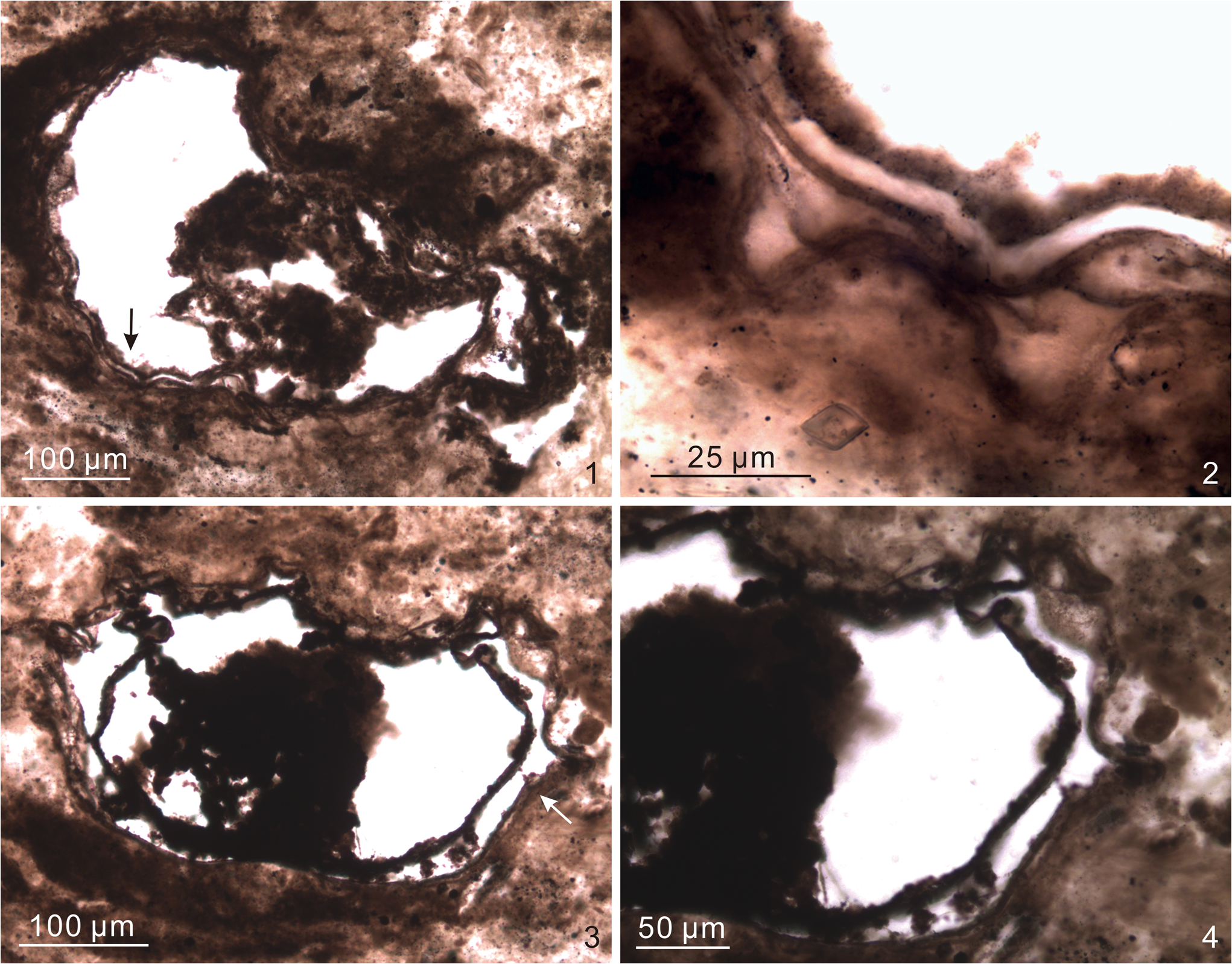
Figure 18. Asterocapsoides sinensis. (1, 2) DH-14-65.0-B, 14.3 × 134.6, EF-N34-4, VPIGM-4833, arrow in (1) marks area shown in (2) at a different focal level; (3, 4) DH-14-65.0-B, 15.8 × 132.0, EF-M31-2, VPIGM-4834, arrow in (3) marks area shown in (4).
Neotype
The holotype designated by Yin and Li (Reference Yin and Li1978) was damaged and a neotype was subsequently designated by Zhang et al. (Reference Zhang, Yin, Xiao and Knoll1998). The neotype is reposited in the Nanjing Institute of Geology and Palaeontology (thin section R-19-3; Zhang et al., Reference Zhang, Yin, Xiao and Knoll1998, fig. 5.10).
Occurrence
Ediacaran of South China, northern India, and Russia. South China: Doushantuo Formation at Tianzhushan, Changyang, Hubei Province (Yin and Li, Reference Yin and Li1978; Zhang et al., Reference Zhang, Yin, Xiao and Knoll1998); upper Doushantuo Formation at Chaoyang, Shangrao, Jiangxi Province (Zhou et al., Reference Zhou, Chen and Xue2002); Doushantuo Formation at Wangfenggang, Yangtze Gorges area, Hubei Province (Yin et al., Reference Yin, Liu, Gao, Wang, Tang and Liu2007); member III of Doushantuo Formation at Wangfenggang and Niuping, Yangtze Gorges area, Hubei Province (Liu et al., Reference Liu, Xiao, Yin, Chen, Zhou and Li2014a); member II of Doushantuo Formation at Siduping, Hunan Province (Hawkins et al., Reference Hawkins, Xiao, Jiang, Wang and Shi2017); lower Doushantuo Formation (equivalent to unit 4 at Weng'an or upper member II in the Yangtze Gorges area) at Wanjiagou section, Zhangcunping, Hubei Province (Liu et al., Reference Liu, Yin, Gao, Tang and Chen2009); Doushantuo Formation at Weng'an, Guizhou Province (Yuan et al., Reference Yuan, Xiao, Yin, Knoll, Zhou and Mu2002; Xiao et al., Reference Xiao, Zhou, Liu, Wang and Yuan2014). Northern India: Krol A Formation, Solan area (this paper).
Veis et al. (Reference Veis, Vorob'Eva and Golubkova2006) and Vorob'Eva et al. (Reference Vorob'Eva, Sergeev and Semikhatov2006) mentioned the occurrence of Asterocapsoides sinensis in the Ediacaran Vychegda Formation in the Timan Ridge, East European Platform, Russia, but did not provide illustrations. Subsequently, Vorob'Eva et al. (Reference Vorob'Eva, Sergeev and Knoll2009) illustrated two specimens as Asterocapsoides sp., one of which, in our opinion, can be assigned to A. sinensis (see Remarks below). However, A. sinensis specimens from the Scotia Group of Svalbard (Knoll, Reference Knoll1992) and the Infra-Krol and Krol A formations in northern India (Tiwari and Pant, Reference Tiwari and Pant2004; Sharma et al., Reference Sharma, Kumar, Tiwari, Shukla, Pandey, Srivastava and Banerjee2012, Reference Sharma, Shukla and Sergeev2021, fig. 9B) have acutely conical processes and their identification as A. sinensis remains uncertain (see Remarks below).
Description and measurements
Large spheroidal vesicles with sparsely distributed processes open to vesicle interior. Processes conical, often obtuse, and basally separate from each other. An inner wall is present within the vesicle, and remnants of an outer membrane also may be present. Vesicle diameter 300–400 μm, only a few (<10) processes per circumferential view, process spacing 12 μm or more at base, process width ~30 μm at base, process length 20–40 μm (5–14% of vesicle diameter).
Materials
Two poorly preserved specimens illustrated in Figure 18.
Remarks
One could conceivably argue that the sparse processes in the specimens illustrated in Figure 18 may be deformation artifacts. Indeed, both specimens in our collection are deformed, particularly the inner wall. However, the processes on the vesicle wall do not coincide spatially with the deformation in the inner wall, leading us to favor the interpretation that the processes are biological structures rather than deformational folds of the vesicle wall. If our interpretation is correct, then the Krol A specimens best fit Asterocapsoides sinensis on the basis of their large vesicle size, the presence of an inner wall, as well as sparse, widely separate, and mostly obtusely conical processes.
Two specimens of Asterocapsoides sp. (Zhou et al., Reference Zhou, Chen and Xue2002, pl. 2, fig. 6; Vorob'Eva et al., Reference Vorob'Eva, Sergeev and Knoll2009, fig. 7.10) from the Doushantuo Formation in South China and the Vychegda Formation in Russia also display these features, and thus can be regarded as A. sinensis. On the other hand, specimens of A. sinensis from the Infra-Krol Formation (Tiwari and Pant, Reference Tiwari and Pant2004; Sharma et al., Reference Sharma, Kumar, Tiwari, Shukla, Pandey, Srivastava and Banerjee2012) and Krol A Formation in northern India (Sharma et al., Reference Sharma, Shukla and Sergeev2021, fig. 9B) have basally separate and acutely conical processes, as do specimens of Asterocapsoides sp. from the same formation (Tiwari and Knoll, Reference Tiwari and Knoll1994; Tiwari and Pant, Reference Tiwari and Pant2004); these may be either A. robustus or A. wenganensis. One of the Krol A specimens illustrated as A. sinensis in Sharma et al. (Reference Sharma, Shukla and Sergeev2021, fig. 9C) is poorly preserved and does not exhibit diagnostic features of this species. Finally, the specimen illustrated as A. sinensis from the Scotia Group of Svalbard (Knoll, Reference Knoll1992) has sparsely distributed, basally separate, and acutely conical processes that seem to be divided internally by transverse septa; this specimen is akin to A. wenganensis or Weissiella grandistella Vorob'Eva et al., Reference Vorob'Eva, Sergeev and Knoll2009, depending on future verification of the presence of transverse septa within processes.
Genus Cavaspina Moczydłowska, Vidal, and Rudavskaya, Reference Moczydłowska, Vidal and Rudavskaya1993
Type species
Cavaspina acuminata (Kolosova, Reference Kolosova1991) Moczydłowska, Vidal, and Rudavskaya, Reference Moczydłowska, Vidal and Rudavskaya1993.
Other species
Cavaspina basiconica Moczydłowska, Vidal, and Rudavskaya, Reference Moczydłowska, Vidal and Rudavskaya1993; C. conica Liu and Moczydłowska, Reference Liu and Moczydłowska2019; C. uria (Nagovitsin and Faizullin in Nagovitsin et al., Reference Nagovitsin, Faizullin and Yakshin2004) Nagovitsin and Moczydłowska in Moczydłowska and Nagovitsin, Reference Moczydłowska and Nagovitsin2012.
Remarks
Liu and Moczydłowska (Reference Liu and Moczydłowska2019) regarded Cavaspina amplitudinis Willman in Willman and Moczydłowska, Reference Willman and Moczydłowska2011, as a junior synonym of Appendisphaera tenuis Moczydłowska, Vidal, and Rudavskaya, Reference Moczydłowska, Vidal and Rudavskaya1993.
Cavaspina tiwariae Xiao new species
Figure 19
- Reference Shukla and Tiwari2014
Unnamed Form A, Shukla and Tiwari, p. 219, fig. 6C, D.

Figure 19. Cavaspina tiwariae Xiao new species. (1–4) Holotype, DH-14-65.0-A, 14.5 × 130.0, EF-N30-1, VPIGM-4830, (2–4) show the same area indicated by the arrow in (1) at different focal levels.
Holotype
VPIGM-4830, thin section DH-14-65.0-A, Olympus BX-51 coordinates 14.5 × 130.0, England Finder coordinates N30-1, illustrated in Figure 19, reposited in Museum of Geosciences at Virginia Tech, from Krol A Formation in Solan area, Lesser Himalaya, northern India.
Diagnosis
A species of Cavaspina with a medium-sized vesicle bearing sparse, deflated, and obtusely conical processes.
Occurrence
Thus far only known from the Ediacaran Krol A Formation in the Solan area, Lesser Himalaya, northern India (Shukla and Tiwari, Reference Shukla and Tiwari2014; this paper).
Description and measurements
Medium-sized spherical vesicles with sparsely distributed and widely separate processes that are short, deflated, obtusely conical, and open to vesicle interior. The transition from processes to vesicle wall is gradual. Vesicle diameter ~150 μm, fewer than 20 processes per circumferential view, process spacing 19–28 μm at apex (spacing at base is difficult to measure because the transition from processes to vesicle wall is gradual), process width up to 8 μm at base, process length up to 5 μm (~3% of vesicle diameter).
Etymology
In honor of Dr. Meera Tiwari, who pioneered the study of microfossils from the Infra-Krol and Krol A formations in the Lesser Himalaya and published a specimen that is here regarded as conspecific to this new species (Shukla and Tiwari, Reference Shukla and Tiwari2014, fig. 6C, D).
Material
One illustrated specimen, the holotype (Fig. 19) and a previously published specimen from the Krol A Formation in the Solan area (Shukla and Tiwari, Reference Shukla and Tiwari2014, fig. 6C, D).
Remarks
The specimen illustrated here is somewhat similar to Asterocapsoides sinensis (Fig. 18) in its sparsely distributed, relatively short, and obtusely conical processes. However, it can be distinguished by its deflated processes, much smaller vesicle size, and the lack of an inner wall and an outer wall, which are thought to be diagnostic of A. sinensis (Liu and Moczydłowska, Reference Liu and Moczydłowska2019), although Xiao et al. (Reference Xiao, Zhou, Liu, Wang and Yuan2014) pointed out that these additional walls (particularly the inner wall) could be lost during diagenesis. This specimen is also similar to Polygonium sp. of Liu et al. (Reference Liu, Xiao, Yin, Chen, Zhou and Li2014a) in its sparsely distributed processes that gradually transition at base to vesicle wall, but its processes are much smaller than those of the latter species. It better fits the genus Cavaspina, which is characterized by relatively short and sparsely distributed processes that are generally conical in shape (Moczydłowska et al., Reference Moczydłowska, Vidal and Rudavskaya1993). In particular, it is similar to the type species of Cavaspina, C. acuminata, in its sparsely distributed, relatively short, and conical processes. However, the Krol A specimen can be differentiated by its larger vesicle as well as its deflated and obtusely conical processes, which are distinct from the acutely conical processes (1 μm wide and 3–5 μm long) of C. acuminata. The Krol A specimen is also somewhat similar to Cavaspina uria in process size, but the latter species has a smaller vesicle size (80–130 μm), acutely conical processes (5–11 μm long and 4–12 μm wide at base), and more closely spaced processes (>15 processes in circumferential view; estimated from Moczydłowska and Nagovitsin, Reference Moczydłowska and Nagovitsin2012, fig. 4G, I). More importantly, the processes of C. uria are not deflated. Thus, a new species is erected here on the basis the specimen illustrated in Figure 19 and a morphologically similar specimen previously published from the same stratigraphic unit in the same area (Shukla and Tiwari, Reference Shukla and Tiwari2014).
Genus Cymatiosphaeroides Knoll, Reference Knoll1984, emend. Shang et al., Reference Shang, Liu and Moczydłowska2019
Type species
Cymatiosphaeroides kullingii Knoll, Reference Knoll1984, emend. Shang et al., Reference Shang, Liu and Moczydłowska2019
Other species
Cymatiosphaeroides forabilatus Liu and Moczydłowska, Reference Liu and Moczydłowska2019; C. yinii Yuan and Hofmann, Reference Yuan and Hofmann1998.
Remarks
Cymatiosphaeroides dilutopilum Zang in Zang and Walter (Reference Zang and Walter1992) and C. pilatopilum Zang in Zang and Walter (Reference Zang and Walter1992) were synonymized and transferred to Appendisphaera dilutopila (Zang in Zang and Walter, Reference Zang and Walter1992) Grey, Reference Grey2005, although some non-holotype specimens illustrated in Zang and Walter (Reference Zang and Walter1992) as C. dilutopilum and C. pilatopilum have been reassigned by Grey (Reference Grey2005) to Appendisphaera barbata and Knollisphaeridium triangulum (Zang in Zang and Walter, Reference Zang and Walter1992) Willman and Moczydłowska, Reference Willman and Moczydłowska2008, respectively. More recently, Liu and Moczydłowska (Reference Liu and Moczydłowska2019, p. 61) considered Appendisphaera dilutopila as a synonym of Appendisphaera tabifica.
The genus Cymatiosphaeroides was originally diagnosed as a double-walled acanthomorph with thin and solid processes arising from the inner wall and supporting the outer wall (Knoll, Reference Knoll1984). It was later emended to emphasize that the outer wall can be a single-layered or multilamellate structure (Knoll et al., Reference Knoll, Swett and Mark1991). The diagnosis was recently emended again by Shang et al. (Reference Shang, Liu and Moczydłowska2019), who diagnosed the genus Cymatiosphaeroides as a double-walled acanthomorph with thin, hollow, and cylindrical processes. It remains to be verified whether the holotype of the type species, C. kullingii, has hollow processes; this feature cannot be determined with confidence from the published illustrations (Knoll, Reference Knoll1984, fig. 9A, B) because the processes are extremely thin (~1 μm in width). Nonetheless, we follow the emendation of Shang et al. (Reference Shang, Liu and Moczydłowska2019) so that C. forabilatus, which has hollow processes (see below), can be included in the genus Cymatiosphaeroides.
The genus Cymatiosphaeroides and its type species, C. kullingii, have extremely long stratigraphic distributions. The oldest known occurrences of C. kullingii are from the ~1.6 Ga Chitrakoot Formation in the Vindhyan Basin, central India (Anbarasu, Reference Anbarasu2001; Singh and Sharma, Reference Singh and Sharma2014). Specimens identified as Shuiyousphaeridium echinulatum Yin and Gao, Reference Yin and Gao1999, from the Chitrakoot Formation (Singh and Sharma, Reference Singh and Sharma2014) have been reassigned to C. kullingii by Liu and Moczydłowska (Reference Liu and Moczydłowska2019). Examples of Tonian C. kullingii are from the Svanbergfjellet and Draken formations of the Akademikerbreen Group in northeastern Svalbard (Knoll et al., Reference Knoll, Swett and Mark1991; Butterfield et al., Reference Butterfield, Knoll and Swett1994), the Fifteenmile Group in Northwest Canada (Allison and Awramik, Reference Allison and Awramik1989), and the Chuar Group in the Grand Canyon of the western U.S. (Vidal and Ford, Reference Vidal and Ford1985). Examples of Ediacaran Cymatiosphaeroides include C. kullingii from the Doushantuo Formation in South China (see Liu and Moczydłowska, Reference Liu and Moczydłowska2019, for details).
Cymatiosphaeroides forabilatus Liu and Moczydłowska, Reference Liu and Moczydłowska2019, emend. Shang et al., Reference Shang, Liu and Moczydłowska2019
Figures 20–22
- Reference Tiwari and Azmi1992
Form B, Tiwari and Azmi, p. 390, pl. 1, fig. 15.
- Reference Tiwari and Knoll1994
Ericiasphaera spjeldnaesii Vidal, Reference Vidal1990; Tiwari and Knoll, p. 198, pl. 1, fig. 1 (part).
- Reference Tiwari and Knoll1994
Unclassified acanthomorphic acritarch; Tiwari and Knoll, p. 198, pl. 1, fig. 6.
- Reference Shukla and Tiwari2014
Unnamed Form E; Shukla and Tiwari, p. 222, fig. 7E–G.
- Reference Liu and Moczydłowska2019
Cymatiosphaeroides forabilatus Liu and Moczydłowska, p. 81, fig. 41.
- Reference Shang, Liu and Moczydłowska2019
Cymatiosphaeroides forabilatus; emend. Shang et al., p. 22, figs. 9, 10A–C.

Figure 20. Cymatiosphaeroides forabilatus. (1–4) DH-14-67.0-C, 11.8 × 139.0, EF-Q39-1, VPIGM-4848, white rectangle in (1) marks area shown in (2) at a different focal level, and black rectangle in (1) marks area shown in (3, 4) at two different focal levels; (5–7) DH-14-67.0-C-2, 12.0 × 135.9, EF-Q36-3, VPIGM-4852, rectangle in (5) marks area shown in (6) at a different focal level, and arrow in (6) marks area shown in (7) at a different focal level and with a rotation; (8–11) DH-14-67.0-C-2, 15.2 × 137.3, EF-N37, VPIGM-4854, white and black rectangles in (8) mark areas shown in (9) and (11), respectively, and arrow in (9) marks area magnified in (10).

Figure 21. Cymatiosphaeroides forabilatus. (1–4) DH-14-67.0-B-2, 12.5 × 136.7, EF-P37-3, VPIGM-4846, rectangle in (1) marks area shown in (2), and arrow in (2) marks area shown in (3, 4) at different focal levels; (5–7) DH-14-67.0-C-2, 15.3 × 137.7, EF-M37-3, VPIGM-4855, rectangles in (5, 6) mark areas shown in (6, 7), respectively; (8–10) S4-4-F2-5, 8.4 × 128.8, EF-U29-1, VPIGM-4876, white and black rectangles in (8) mark areas shown in (9, 10), respectively, at a different focal levels.

Figure 22. Cymatiosphaeroides forabilatus. (1–3) S4-4-F2-15, 10.3 × 129.7, EF-S30-1, VPIGM-4898, rectangle in (1) marks area shown in (2) with 180° rotation, and arrow in (2) marks area shown in (3); (4, 5) S4-4-F2-15, 22.3 × 106.4, EF-F6, VPIGM-4902, rectangle in (4) marks area shown in (5); (6, 7) S4-4-F2-14, 22.8 × 134.7, EF-E34-4, VPIGM-4897, arrow in (6) marks area shown in (7); (8–10) S4-4-F2-9, 16.4 × 135.5, EF-M35, VPIGM-4895, rectangle in (8) marks area shown in (9), and arrow in (9) marks area shown in (10).
Holotype
IGCAGS–D2XFH212, thin section XFH0946-1-10, reposited at the Institute of Geology, Chinese Academy of Geological Sciences, from member II of the Ediacaran Doushantuo Formation at Xiaofenghe section in the Yangtze Gorges area, Hubei Province, South China (Liu and Moczydłowska, Reference Liu and Moczydłowska2019, fig. 41A, B).
Occurrence
Ediacaran of South China and northern India. South China: member II of Doushantuo Formation at northern and southern Xiaofenghe sections, Yangtze Gorges area, Hubei Province (Liu and Moczydłowska, Reference Liu and Moczydłowska2019); Doushantuo Formation at Liujing section in Guizhou Province (Shang et al., Reference Shang, Liu and Moczydłowska2019). Northern India: Krol A Formation, Solan area (this paper).
Description and measurements
Large double-walled spheroidal vesicles with densely and evenly distributed processes. Processes arise from the inner wall and penetrate the outer wall. They are thin, short, uniform in length, and basally separate. Processes are apparently solid at the distal end, but many of them are hollow at least at the base, with a small basal expansion tapering distally toward a thin filament (e.g., Fig. 20.2–20.4). Vesicle diameter ~315–430 μm (estimated from Fig. 20.1, 20.5, 20.8), 28–41 processes per 100 μm of vesicle periphery, process length 6–10 μm (~1.5–2.4% of vesicle diameter), process spacing 1–3 μm at base, basal expansion (when discernable) 1–2 μm wide at base and 1–2 μm in height, apical spine ~0.5–0.9 μm wide and 5–7 μm long. Inner and outer walls ~5 μm apart.
Remarks
The Krol A specimens are identified as Cymatiosphaeroides forabilatus based on their double-walled vesicles that bear processes arising from the inner wall and penetrating the outer wall. Processes are extremely thin and it is difficult to determine whether they are solid or hollow. However, in several better-preserved specimens, it can be seen that the processes have a small basal expansion and is hollow at least at the basal part (e.g., Fig. 20.2–20.4). Shang et al. (Reference Shang, Liu and Moczydłowska2019) also noted that the processes of C. forabilatus are “slightly widened at the bases” in some specimens and that they are hollow in nature.
The specimen illustrated in Figure 22.8–22.10 is strongly degraded and obscured by the accumulation of organic matter. Nonetheless, remnants of processes extruding beyond the outer wall can be seen in the upper left of Figure 22.10. Thus, we regard this specimen as a poorly preserved example of C. forabilatus.
In sharp contrast to Cymatiosphaeroides kullingii, which has an extremely long stratigraphic range, C. forabilatus is restricted to the Ediacaran based on biostratigraphic data available thus far. Indeed, this is one of the common species in the Ediacaran Doushantuo Formation in South China and Infra-Krol–Krol A formations in northern India. Several previously published specimens from the Infra-Krol–Krol A formations are characterized by large double-walled vesicles with thin processes arising from the inner wall and penetrating the outer wall. These specimens are similar to the specimens described here in vesicle size, process length and width, and spacing between double walls, and some of them have a small expansion at the base of the processes (e.g., Shukla and Tiwari, Reference Shukla and Tiwari2014). These specimens include (1) Form B in Tiwari and Azmi (Reference Tiwari and Azmi1992), which was subsequently described as Ericiasphaera spjeldnaesii in Tiwari and Knoll (Reference Tiwari and Knoll1994); (2) Unclassified acanthomorphic acritarch in Tiwari and Knoll (Reference Tiwari and Knoll1994), which was later assigned to Tianzhushania spinosa (Zhang et al., Reference Zhang, Yin, Xiao and Knoll1998); and (3) Unnamed Form E of Shukla and Tiwari (Reference Shukla and Tiwari2014). These specimens are here considered to be Cymatiosphaeroides forabilatus. Additionally, specimens illustrated as Tianzhushania spinosa (Shukla et al., Reference Shukla, Mathur, Babu and Srivastava2008, pl. 3, figs. 1, 2) and Echinosphaeridium maximum (Shukla et al., Reference Shukla, Mathur, Babu and Srivastava2008, pl. 2, figs. 13, 14) appear to have double-walled vesicles bearing processes that arise on the inner wall and penetrate the outer wall; these may also be C. forabilatus, but further examination is needed to verify this suspicion because the specimens are poorly preserved.
Genus Dictyotidium Eisenack, Reference Eisenack1955, emend. Staplin, Reference Staplin1961
Type species
Dictyotidium dictyotum Eisenack, Reference Eisenack1955
Other species
Two additional species have been reported from the Precambrian: Dictyotidium fullerene Butterfield in Butterfield et al. (Reference Butterfield, Knoll and Swett1994) from the Tonian Algal Dolomite Member of the Svanbergfjellet Formation at Geerabukta of Spitsbergen, and Dictyotidium ambonum Zang in Zang and Walter (Reference Zang and Walter1992) from the Ediacaran Pertatataka Formation at Rodinga 4 drill core in Amadeus Basin, central Australia. Other species are listed in Eisenack et al. (Reference Eisenack, Cramer and Díez1979) and Fensome et al. (Reference Fensome, Williams, Barss, Frerman and Hill1990).
Dictyotidium grazhdankinii Xiao new species
Figure 23
Holotype
VPIGM-4832, thin section DH-14-65.0-A-2, Olympus BX-51 coordinates 10.6 × 108.8, England Finder coordinates R9-3, illustrated in Figure 23.1–23.5, reposited in Museum of Geosciences at Virginia Tech, from Krol A Formation in Solan area, Lesser Himalaya, northern India.

Figure 23. Dictyotidium grazhdankinii Xiao new species. (1–5) Holotype, DH-14-65.0-A-2, 10.6 × 108.8, EF-R9-3, VPIGM-4832, arrow in (1) marks area shown in (2–4) at different focal levels, and arrow in (3) marks area magnified in (5); (6, 7) S4-4-F2-5-2, 11.9 × 128.5, EF-Q28-4, VPIGM-4881, arrow in (6) marks area magnified in (7).
Diagnosis
A species of Dictyotidium with a delicate network consisting of polygonal reticular fields that are 1–5 μm in dimension and defined by thin ridges 0.2–1.0 μm in thickness. No processes are present.
Occurrence
Ediacaran Krol A Formation in the Solan area, Lesser Himalaya, northern India (this paper).
Description and measurements
Medium-sized to large vesicles consisting of a reticulate framework with apparent absence of a continuous vesicle wall. Vesicle diameter 100–400 μm (holotype 120 μm, Fig. 23.1). Polygonal fields 1–5 μm in size (2–4 μm in holotype, Fig. 23.2–23.5). Ridges 0.2–1.0 μm in thickness (0.2–0.4 μm in holotype, Fig. 23.5).
Etymology
In recognition of Dr. Dmitriy Grazhdankin's contributions to Ediacaran paleontology and his service to the Ediacaran Subcommission.
Materials
Two illustrated specimens (Fig. 23). Additionally, there are as many as 30 poorly preserved specimens that may be this species, although some of them may simply be degraded sphaeromorphs.
Remarks
One may argue that the reticulate pattern of the Krol A specimens is a result of degradation or taphonomic alteration of the vesicle wall. For example, recrystallization of quartz crystals may displace and concentrate organic residues along crystal interfaces to form polygonal crystal rings (Brasier et al., Reference Brasier, McLoughlin, Green and Wacey2006). There is no doubt that Krol A fossils have been subjected to taphonomic processes, including alteration related to crystal growth. This can be seen in numerous cases of discontinuous carbonaceous particles that outline microfossil structures (e.g., processes of Appendisphaera? hemisphaerica, Fig. 8.10). However, close examination of the best-preserved specimens, here identified as Dictyotidium grazhdankinii Xiao n. sp., led us to believe that their reticulate pattern is not a taphonomic artifact. First, observation under cross-polarized light microscopy shows that the fossils are replicated by microcrystalline silica and the reticulate pattern does not match any crystal extinction pattern when observed under crossed nicols. Second, microscopic observation by adjusting the focal level confirms that the organic ridges are one-dimensional thread-like structures that are relatively continuous, uniform in thickness, and weaved into a reticulate sheet (Fig. 23.2–23.4). Importantly, when the fossil is deformed, the reticulate sheet is also folded accordingly, without any disruption of the reticulate pattern (Fig. 23.2–23.4). These observations are in sharp contrast to polygonal crystal rings (Brasier et al., Reference Brasier, McLoughlin, Green and Wacey2006) that form three-dimensional spongy networks consisting of discontinuous carbonaceous particles distributed along crystal interfaces and often are concentrated at multi-crystal junctions (where incongruent crystal morphologies result in open space where organic matter accumulates). Thus, for the best-preserved specimens (e.g., Fig. 23.1–23.5), the regular reticulate morphology is unlikely a taphonomic artifact. In some specimens, parts of the vesicle show a regular reticulate sheet, whereas the other parts have more degraded patterns (e.g., Fig. 23.6, 23.7); these are interpreted as Dictyotidium grazhdankinii Xiao n. sp. specimens that have been unevenly altered by taphonomic processes.
Herkomorphs are acritarchs with their vesicle walls divided into polygonal fields. Examples include Cymatiosphaera Wetzel, Reference Wetzel1933; Dictyosphaera Xing and Liu, Reference Xing and Liu1973; Dictyosphaeridium Wetzel, Reference Wetzel1952; and Dictyotidium Eisenack, Reference Eisenack1955. In Cymatiosphaera, the polygonal fields are defined by fence-like structures perpendicular to the vesicle wall, thus distinct from the reticulate ridges in the Krol A specimens. In the other three genera, the polygonal fields are defined by ridges on the vesicle wall (typically manifested as polygonal platelets in Dictyosphaera; Agić et al., Reference Agić, Moczydłowska and Yin2015). However, Dictyosphaeridium bears processes, and Dictyosphaera may represent a different ontogenetic stage of the acanthomorphic herkomorph Shuiyousphaeridium Yan in Yan and Zhu, Reference Yan and Zhu1992 (Xiao et al., Reference Xiao, Knoll, Kaufman, Yin and Zhang1997; Agić et al., Reference Agić, Moczydłowska and Yin2015). Thus, given its lack of processes, the Krol A specimens are best referred to the genus Dictyotidium.
Dictyotidium grazhdankinii Xiao n. sp. can be distinguished from the other Ediacaran species of Dictyotidium (D. ambonum Zang in Zang and Walter, Reference Zang and Walter1992) by its larger overall size but smaller reticulum size, as well as its apparent lack of a continuous vesicle wall. It is similar to the Tonian species D. fullerene Butterfield in Butterfield et al. (Reference Butterfield, Knoll and Swett1994) in that both taxa apparently lack a continuous vesicle wall. However, D. fullerene has short processes at the corners of polygonal fields and has thicker and more robust ridges. The apparent lack of a continuous vesicle wall is probably a taphonomic artifact; perhaps the vesicle wall was thin and had been preferentially degraded.
A specimen illustrated as “reticulate acanthomorphic acritarch” from the upper Ediacaran or Cambrian Oppokun Formation in north-central Siberia (Grazhdankin et al., Reference Grazhdankin, Nagovitsin, Golubkova, Karlova, Kochnev, Rogov and Marusin2020) also has a vesicle consisting of a reticulate network, and is similar to Dictyotidium grazhdankinii Xiao n. sp. in vesicle size, reticulum size, and ridge thickness. However, the Oppokun specimen seems to have minute and faintly preserved processes.
Genus Mengeosphaera Xiao et al., Reference Xiao, Zhou, Liu, Wang and Yuan2014
Type species
Mengeosphaera chadianensis (Chen and Liu, Reference Chen and Liu1986) Xiao et al., Reference Xiao, Zhou, Liu, Wang and Yuan2014.
Other species
Mengeosphaera angusta Liu et al., Reference Liu, Xiao, Yin, Chen, Zhou and Li2014; M. bellula Liu et al., Reference Liu, Xiao, Yin, Chen, Zhou and Li2014; M. constricta Liu et al., Reference Liu, Xiao, Yin, Chen, Zhou and Li2014; M. eccentrica Xiao et al., Reference Xiao, Zhou, Liu, Wang and Yuan2014; M. flammelata Liu and Moczydłowska, Reference Liu and Moczydłowska2019; M. gracilis Liu et al., Reference Liu, Xiao, Yin, Chen, Zhou and Li2014; M. grandispina Liu et al., Reference Liu, Xiao, Yin, Chen, Zhou and Li2014; M. latibasis Liu et al., Reference Liu, Xiao, Yin, Chen, Zhou and Li2014; M. lunula Liu and Moczydłowska, Reference Liu and Moczydłowska2019; M. matryoshkaformis Ouyang et al., Reference Ouyang, Zhou, Xiao, Guan, Chen, Yuan and Sun2021; M. membranifera Shang et al., Reference Shang, Liu and Moczydłowska2019; M. minima Liu et al., Reference Liu, Xiao, Yin, Chen, Zhou and Li2014; M. reticulata (Xiao and Knoll, Reference Xiao and Knoll1999) Xiao et al., Reference Xiao, Zhou, Liu, Wang and Yuan2014; M. spinula Liu et al., Reference Liu, Xiao, Yin, Chen, Zhou and Li2014; M. stegosauriformis Liu et al., Reference Liu, Xiao, Yin, Chen, Zhou and Li2014; M. uniformis Liu et al., Reference Liu, Xiao, Yin, Chen, Zhou and Li2014.
Remarks
The genus Mengeosphaera is characterized by closely and evenly arranged biform processes (Xiao et al., Reference Xiao, Zhou, Liu, Wang and Yuan2014). However, the definition of biform processes varies. According to Grey (Reference Grey2005, p. 175), a biform process has “a conical base and tapering or ciliate distal portion.” Following this definition, a gradually tapering process (e.g., in Tanarium conoideum Kolosova, Reference Kolosova1991; see simplified diagrams in Fig. 24.2–24.4) would be regarded as biform. Subsequently, a biform process was defined as a process with “a basal expansion and an apical spine or lateral spine, often separated by an inflection point” (Xiao et al., Reference Xiao, Zhou, Liu, Wang and Yuan2014, p. 4), emphasizing the distinct and recognizable boundary between the basal and apical parts of the process (Fig. 24.5–24.10). An inflection point can represent a gradual transition from an inflated basal expansion to a distally tapering or cylindrical apical spine (e.g., Fig. 24.5). This is analogous to the mathematical inflection point where a convex curve changes to a concave curve and where the second derivative of the curve is zero (or where the distally decreasing slope of the inflated basal expansion transitions to the distally increasing slope of the deflated apical spine). From a practical point of view, an inflection point of a biform process also can represent an abrupt change in slope between the basal expansion and apical spine, regardless of whether the basal expansion is inflated (Fig. 24.6–24.10). In contrast, a process with a concave (or deflated) basal expansion continuing into an apical spine without an inflection point in between is not regarded as a biform process (Fig. 24.2–24.4), even if it would fit the definition of Grey (Reference Grey2005).

Figure 24. Schematic illustration of non-biform (1–4) and biform processes (5–10). Arrows point to inflection points in biform processes.
The genus Mengeosphaera was diagnosed by its biform processes with a conical or domical, often inflated, basal expansion that tapers rapidly and supports an apical spine that is acutely conical, often very thin, and tapers gradually (Xiao et al., Reference Xiao, Zhou, Liu, Wang and Yuan2014). An inflection point separates the basal expansion and apical spine. This feature is best seen in the holotype of the type species, Mengeosphaera chadianensis (Chen and Liu, Reference Chen and Liu1986). Xiao et al. (Reference Xiao, Zhou, Liu, Wang and Yuan2014) described two additional species of Mengeosphaera, M. reticulata and M. eccentrica, both of which are characterized by biform processes.
Liu and Moczydłowska (Reference Liu and Moczydłowska2019) commented that the inflated basal expansion of Mengeosphaera processes could be a taphonomic artifact unique to silica or phosphate mineralization, but not in shale preservation. We understand that an originally inflated basal expansion could become deflated due to degradation, contraction, and compression, regardless of preservation mode of mineralization and carbonaceous compression. However, it is difficult to understand how an originally deflated or otherwise non-inflated basal expansion would become consistently inflated during fossil mineralization, particularly when the basal expansion is preserved with structural integrity and show no evidence of organic displacement due to mineral recrystallization. Silicification or phosphatization is fundamentally a process of microcrystal precipitation on organic substrates, resulting in a mold and/or cast of the organic structure (Oehler and Schopf, Reference Oehler and Schopf1971; Xiao and Tang, Reference Xiao and Tang2022). Organic structures such as basal expansions can be preserved with structural integrity when they are coated with or embedded in phosphate and silica. If they are disintegrated or destroyed by mineral recrystallization, organic walls or membranes would be disrupted to form irregular structures. It is unlikely for a deflated basal expansion to become consistently inflated during mineralization/recrystallization and to still maintain its structural integrity.
Liu and Moczydłowska (Reference Liu and Moczydłowska2019) further stated that neither M. reticulata nor M. eccentrica have biform processes. They did not define what they meant by biform processes. Following either of the definitions of Grey (Reference Grey2005) or Xiao et al. (Reference Xiao, Zhou, Liu, Wang and Yuan2014), as clarified above and schematically illustrated in Figure 24, it is indisputable that all three species of Mengeosphaera described in Xiao et al. (Reference Xiao, Zhou, Liu, Wang and Yuan2014) have biform processes with clear inflection points. This key feature is clearly present in the holotypes of M. chadianensis, M. reticulata, and M. eccentrica, illustrated in Chen and Liu (Reference Chen and Liu1986, pl. 2, figs. 2, 4), Xiao and Knoll (Reference Xiao and Knoll1999, fig. 11H), and Xiao et al. (Reference Xiao, Zhou, Liu, Wang and Yuan2014, fig. 26.1), respectively. It should be pointed out that these holotypes are all three-dimensionally phosphatized and acid-extracted specimens that were imaged using scanning electron microscopy so that the biform nature of the processes is best seen in lateral views, but not discernable in apical views. Additionally, the fragile apical spines may be abraded during taphonomic reworking or acid extraction, and they may not be retained at all if the specimens are preserved as internal molds (Xiao and Knoll, Reference Xiao and Knoll1999). Despite these complications, the processes in the holotypes of the above-mentioned species were clearly illustrated, with an inflection point separating an inflated basal expansion and an apical spine, although the distal part of the apical spine is often not intact.
Liu et al. (Reference Liu, Xiao, Yin, Chen, Zhou and Li2014a) established 13 new species of Mengeosphaera, four of which are discussed here because of their uncertain placement in this genus. Mengeosphaera? cuspidata has conical or even deflated basal expansions and, as a result, was published as an open nomenclature to acknowledge its uncertain placement in Mengeosphaera. This species was subsequently transferred to the genus Tanarium Kolosova, Reference Kolosova1991, becoming T. cuspidatum (Liu et al., Reference Liu, Xiao, Yin, Chen, Zhou and Li2014) Liu and Moczydłowska, Reference Liu and Moczydłowska2019. Similarly, Liu et al. (Reference Liu, Xiao, Yin, Chen, Zhou and Li2014a) noted that the basal expansion of M. triangularis was not clearly inflated and this species was tentatively placed in the genus Mengeosphaera. This species also was transferred to the genus Tanarium and became T. triangulare (Liu et al., Reference Liu, Xiao, Yin, Chen, Zhou and Li2014) Liu and Moczydłowska, Reference Liu and Moczydłowska2019, an orthographic correction of T. triangularis as spelled in Liu and Moczydłowska (Reference Liu and Moczydłowska2019, p. 143, 151). In the same paper, however, Liu and Moczydłowska (Reference Liu and Moczydłowska2019, p. 129) listed M. triangularis as an accepted species of Mengeosphaera, likely as an unintended error. Liu and Moczydłowska (Reference Liu and Moczydłowska2019) mentioned in passing that M. spicata Liu et al., Reference Liu, Xiao, Yin, Chen, Zhou and Li2014, is a junior synonym of M. constricta, but provided no explanation or justification. Although the holotypes of these two species are notably different in vesicle size, process density, process spacing, and the presence of a constriction at process base (compare fig. 56.1, 56.2 and fig. 64.1, 64.2 of Liu et al., Reference Liu, Xiao, Yin, Chen, Zhou and Li2014a), we do acknowledge that there are specimens (e.g., Liu et al., Reference Liu, Xiao, Yin, Chen, Zhou and Li2014a, fig. 58) that are morphologically transitional between the two holotypes. Thus, we tentatively follow the synonymization of these two species proposed by Liu and Moczydłowska (Reference Liu and Moczydłowska2019). Finally, the species M.? gracilis was placed in open nomenclature because, relative to other Mengeosphaera species, it has somewhat densely arranged processes with somewhat long and thin apical spines, which are features typically associated with species of Appendisphaera (Liu et al., Reference Liu, Xiao, Yin, Chen, Zhou and Li2014a). Liu and Moczydłowska (Reference Liu and Moczydłowska2019), however, removed the ambiguity in genus placement (although it was still listed as M.? gracilis in Liu and Moczydłowska, Reference Liu and Moczydłowska2019, p. 129), a proposition followed by subsequent authors (e.g., Shang et al., Reference Shang, Liu and Moczydłowska2019) and in this paper.
Mengeosphaera gracilis Liu et al., Reference Liu, Xiao, Yin, Chen, Zhou and Li2014
Figure 25
- Reference Liu, Xiao, Yin, Chen, Zhou and Li2014a
Mengeosphaera? gracilis Liu et al., p. 96, fig. 60.
- Reference Liu and Moczydłowska2019
Mengeosphaera gracilis; Liu and Moczydłowska, p. 132, fig. 71.
- Reference Shang, Liu and Moczydłowska2019
Mengeosphaera gracilis; Shang et al., p. 25, fig. 14F, G.
- Reference Shang and Liu2020
Mengeosphaera gracilis; Shang and Liu, p. 158, fig. 6F–L.
- Reference Ouyang, Zhou, Xiao, Guan, Chen, Yuan and Sun2021
Mengeosphaera gracilis; Ouyang et al., fig. 16K–M.

Figure 25. Mengeosphaera gracilis. (1–3) S4-4-F2-8-2, 12.2 × 108.2, EF-Q8-3, VPIGM-4894, black and white rectangles in (1) mark areas shown in (2, 3), respectively; (4, 5) S4-4-F2-14, 10.9 × 104.4, EF-S4-2, VPIGM-4896, arrow in (4) marks area shown in (5) at a different focal level; (6, 7) S4-4-F1-2, 18.3 × 102.8, EF-K3-3, VPIGM-4869, rectangle in (6) marks area shown in (7) at a different focal level; (8–10) S4-4-F1-4, 9.2 × 129.0, EF-T29-3, VPIGM-4872, white and black arrows in (8) mark areas shown in (9, 10), respectively, at different focal levels.
Holotype
IGCAGS–WFG–727, reposited at Institute of Geology, Chinese Academy of Geological Sciences, from the lower member III of the Ediacaran Doushantuo Formation at Wangfenggang section in the Yangtze Gorges area, Hubei Province, South China (Liu et al., Reference Liu, Xiao, Yin, Chen, Zhou and Li2014a, fig. 60.1, 60.2).
Occurrence
Ediacaran of South China and northern India. South China: member II of Doushantuo Formation at Wuzhishan (Ouyang et al., Reference Ouyang, Zhou, Xiao, Guan, Chen, Yuan and Sun2021), Jiuqunao, Nantuocun, Niuping, Wangfenggang, and northern and southern Xiaofenghe sections (Liu and Moczydłowska, Reference Liu and Moczydłowska2019), as well as member III of Doushantuo Formation at Wangfenggang, Niuping, Xiaofenghe, Baiguoyuan, Chenjiayuanzi, Dishuiyan, and Liuhuiwan sections, Yangtze Gorges area, Hubei Province (Liu et al., Reference Liu, Xiao, Yin, Chen, Zhou and Li2014a); lower Doushantuo Formation (probably equivalent to member II) at Tianping section, Hunan Province (Shang and Liu, Reference Shang and Liu2020); Doushantuo Formation at Liujing section in Guizhou Province (Shang et al., Reference Shang, Liu and Moczydłowska2019). Northern India: Krol A Formation, Solan area (this paper).
Description and measurements
Large spheroidal vesicles with densely and evenly distributed processes open to vesicle interior. Processes biform, with a conical to slightly inflated basal expansion supporting a thin and distally tapering apical spine. Vesicle diameter ~280 μm (Fig. 25.8), 13–18 processes per 100 μm of vesicle periphery, process length 6–14 μm (~5% of vesicle diameter, as estimated from specimen illustrated in Fig. 25.8), process spacing 1–3 μm at base, basal expansion 4–6 μm wide at base, and 3–4 μm in height, apical spine ~1 μm wide and 4–10 μm long.
Materials
Four illustrated specimens (Fig. 25) and three additional specimens.
Remarks
Liu et al. (Reference Liu, Xiao, Yin, Chen, Zhou and Li2014a) commented on the similarities and differences among M. gracilis, Cavaspina basiconica, and Appendisphaera? hemisphaerica, all of which are characterized by processes with a basal expansion supporting a thin apical spine. The processes of C. basiconica do not have an inflated basal expansion and typically are shorter than the two other species. Relative to M. gracilis, A.? hemisphaerica was said to have narrower and more-inflated basal expansion, as well as proportionally longer and more densely arranged processes. As discussed under A.? hemisphaerica, however, these two species may be synonymous. The specimen of M. gracilis illustrated in Shang and Liu (Reference Shang and Liu2020) is poorly preserved, with its biform processes barely visible; therefore its taxonomic identification is tentative.
Genus Tanarium Kolosova, Reference Kolosova1991, emend. Moczydłowska, Vidal, and Rudavskaya, Reference Moczydłowska, Vidal and Rudavskaya1993
Type species
Tanarium conoideum Kolosova, Reference Kolosova1991, emend. Moczydłowska, Vidal, and Rudavskaya, Reference Moczydłowska, Vidal and Rudavskaya1993.
Other species
Tanarium acus Liu et al., Reference Liu, Xiao, Yin, Chen, Zhou and Li2014; T. araithekum Grey, Reference Grey2005; T. capitatum Liu and Moczydłowska, Reference Liu and Moczydłowska2019; T. cuspidatum (Liu et al., Reference Liu, Xiao, Yin, Chen, Zhou and Li2014) Liu and Moczydłowska, Reference Liu and Moczydłowska2019; T. digitiforme (Nagovitsin and Faizullin in Nagovitsin et al., Reference Nagovitsin, Faizullin and Yakshin2004) Sergeev et al., Reference Sergeev, Knoll and Vorob'Eva2011; T. elegans Liu et al., Reference Liu, Xiao, Yin, Chen, Zhou and Li2014; T. gracilentum (Yin in Yin and Liu, Reference Yin, Liu, Zhao, Xing, Ding, Liu, Zhao, Zhang, Meng, Yin, Ning and Han1988) Ouyang et al., Reference Ouyang, Zhou, Xiao, Guan, Chen, Yuan and Sun2021; T. irregulare Moczydłowska, Vidal, and Rudavskaya, Reference Moczydłowska, Vidal and Rudavskaya1993; T. mattoides Grey, Reference Grey2005; T. megaconicum Grey, Reference Grey2005; T.? minimum Liu et al., Reference Liu, Xiao, Yin, Chen, Zhou and Li2014; T.? muntense Grey, Reference Grey2005; Tanarium obesum Liu et al., Reference Liu, Xiao, Yin, Chen, Zhou and Li2014; T. paucispinosum Grey, Reference Grey2005; T. pilosiusculum Vorob'Eva, Sergeev, and Knoll, Reference Vorob'Eva, Sergeev and Knoll2009; T. pluriprotensum Grey, Reference Grey2005; T. pycnacanthum Grey, Reference Grey2005; T. triangulare (Liu et al., Reference Liu, Xiao, Yin, Chen, Zhou and Li2014) Liu and Moczydłowska, Reference Liu and Moczydłowska2019, an orthographic correction of T. triangularis as published in Liu and Moczydłowska (Reference Liu and Moczydłowska2019); T. tuberosum Moczydłowska, Vidal, and Rudavskaya, Reference Moczydłowska, Vidal and Rudavskaya1993; T. uniformum Liu and Moczydłowska, Reference Liu and Moczydłowska2019; T. varium Liu et al., Reference Liu, Xiao, Yin, Chen, Zhou and Li2014; T. victor Xiao et al., Reference Xiao, Zhou, Liu, Wang and Yuan2014.
Remarks
Together with Appendisphaera and Mengeosphaera, Tanarium is one of the richly speciose genera of Ediacaran acanthomorphs. This is due to the very broad definition of this genus. For example, according to Moczydłowska et al.'s (Reference Moczydłowska, Vidal and Rudavskaya1993) emendation, Tanarium is an acanthomorph with hollow processes that are conical or cylindrical, tapering or rounded distally, simple or branching. A number of acanthomorphs—including Papillomembrana Spjeldnaes, Reference Spjeldnaes1963, and Xenosphaera Yin, Reference Yin1987 (see Liu et al., Reference Liu, Xiao, Yin, Chen, Zhou and Li2014a)—would fit in this definition, in which case Tanarium would be rendered a junior synonym. Grey's (Reference Grey2005) emendation restricts Tanarium to acanthomorphs with heteromorphic processes longer than 20% of vesicle diameter. This restriction would exclude a few species, including T.? minimum and T. pilosiusculum Vorob'Eva et al., Reference Vorob'Eva, Sergeev and Knoll2009, from the genus of Tanarium. But even this restriction would still include Xenosphaera and its type species, X. liantuoensis Yin, Reference Yin1987, rendering Tanarium a junior synonym. It is probably time to split the genus Tanarium as currently recognized into several genera on the basis of, for example, process length and morphologies.
Moczydłowska and Nagovitsin (Reference Moczydłowska and Nagovitsin2012) listed Tanarium stellatum Nagovitsin and Faizullin in Nagovitsin et al., Reference Nagovitsin, Faizullin and Yakshin2004, as a junior synonym of T. tuberosum, and Liu and Moczydłowska (Reference Liu and Moczydłowska2019) listed Tanarium obesum Liu et al., Reference Liu, Xiao, Yin, Chen, Zhou and Li2014, as a junior synonym of T. tuberosum, but no explanation was provided to justify these synonymization proposals. Liu et al. (Reference Liu, Xiao, Yin, Chen, Zhou and Li2014a, p. 113) diagnosed T. obesum as a species of Tanarium “with a small to medium-sized vesicle covered with a moderate number of relatively large, acutely conical, and heteromorphic processes that occasionally bifurcate.” Additionally, T. obesum has more numerous, more closely arranged, and more acutely conical processes than does T. tuberosum. In this paper, we follow Liu et al. (Reference Liu, Xiao, Yin, Chen, Zhou and Li2014a) and treat T. obesum as a distinct species (see also Ouyang et al., Reference Ouyang, Zhou, Xiao, Guan, Chen, Yuan and Sun2021).
Tanarium cf. T. conoideum Kolosova, Reference Kolosova1991, emend. Moczydłowska, Vidal, and Rudavskaya, Reference Moczydłowska, Vidal and Rudavskaya1993
Figure 26
- cf. Reference Kolosova1991
Tanarium conoideum Kolosova, p. 57, fig. 5.1–15.3.
- cf. Reference Moczydłowska, Vidal and Rudavskaya1993
Tanarium conoideum; emend. Moczydłowska et al., p. 514, text-fig. 10C, D.

Figure 26. Tanarium cf. T. conoideum. S4-4-F2-7, 17.1 × 120.0, EF-L20-3, VPIGM-4888. (1) Specimen overview; (2–6) close-up views showing details of processes. White and black arrows in (1) mark areas magnified in (2) and (3–6) (same area at different focal levels), respectively. Arrows in (4, 5) mark possible biform processes with a basal expansion.
Occurrence
Ediacaran Krol A Formation, Solan area, Lesser Himalaya, northern India (this paper).
Description and measurements
A poorly preserved specimen with numerous long and conical processes. Estimated maximum vesicle diameter 247 μm (Fig. 26.1), process length up to 60 μm (~24% of vesicle diameter), and process basal width up to 7 μm. Some processes appear to be biform (Fig. 26.4, 26.5), with a basal expansion 14 μm wide and 8 μm high, supporting an apical spine 4 μm in basal width and 45 μm in length.
Materials
One specimen illustrated in Figure 26.
Remarks
The specimen is similar to the holotype of Tanarium conoideum in morphology and proportional length of processes (cf., Kolosova, Reference Kolosova1991, fig. 5.1, 5.2). It is about twice as large in vesicle diameter and its process density is greater than the holotype (although Kolosova, Reference Kolosova1991, illustrated another specimen of T. conoideum with a greater density of processes than in the holotype). Some processes in the current specimen appear to be biform in shape, a feature that is not present in the holotype. Alternatively, these apparently biform processes may be a taphonomic artifact; a torn and dislodged process with its base attached to a small piece of the vesicle wall may appear to be biform. On the other hand, Grey (Reference Grey2005) emended the diagnosis of the genus Tanarium to emphasize its heteromorphic (morphologically variable) processes that are longer than 20% of vesicle diameter. She also commented that the processes of the Australian specimens of T. conoideum have a conspicuously widened base (e.g., Grey, Reference Grey2005, fig. 212D), although she did not specifically describe their processes as biform. Considering the uncertainty about the biform nature of the processes in the only available specimen from the Krol A Formation, we tentatively place this specimen in an open nomenclature. An alternative taxonomic home for this specimen would be the genus Mengeosphaera, if its biform processes can be confirmed with better-preserved specimens that show the intact transition from process base to vesicle wall.
Tanarium digitiforme (Nagovitsin and Faizullin in Nagovitsin et al., Reference Nagovitsin, Faizullin and Yakshin2004) Sergeev et al., Reference Sergeev, Knoll and Vorob'Eva2011
Figure 27
- Reference Nagovitsin, Faizullin and Yakshin2004
Goniosphaeridium digitiforme Nagovitsin and Faizullin in Nagovitsin et al., p. 13, pl. 2, figs. 4, 5.
- Reference Vorob'Eva, Sergeev and Chumakov2008
Unnamed form with processes; Vorob'Eva et al., fig. 2h.
- Reference Golubkova, Raevskaya and Kuznetsov2010
“Goniosphaeridium” digitiforme; Golubkova et al., pl. 4, fig. 3.
- Reference Sergeev, Knoll and Vorob'Eva2011
Tanarium digitiformum (Nagovitsin and Faizullin in Nagovitsin et al., Reference Nagovitsin, Faizullin and Yakshin2004) Sergeev et al., p. 1006, fig. 7.6.
- Reference Moczydłowska and Nagovitsin2012
Tanarium digitiformum; Moczydłowska and Nagovitsin, p. 19, fig. 8D–8F.
- Reference Xiao, Zhou, Liu, Wang and Yuan2014
- Reference Yang, Pang, Chen, Zhong and Yang2020
Tanarium digitiforme; Yang et al., p. 7, fig. 2L–M.

Figure 27. Tanarium digitiforme (Nagovitsin and Faizullin in Nagovitsin et al., Reference Nagovitsin, Faizullin and Yakshin2004) Sergeev et al., Reference Sergeev, Knoll and Vorob'Eva2011. (1, 2) DH-14-68.0-A, 21.3 × 130.3, EF-G30-1, VPIGM-4859, arrow in (1) marks area shown in (2), showing obliquely cut processes; (3–8) DH-14-65.0-A, 16.7 × 109.9, EF-L10-3, VPIGM-4831, (3, 4) the same area at two different focal levels, black and white arrows in (3) mark areas shown in (5) and (7), respectively, and black and white arrows in (4) mark areas shown in (6) and (8), respectively.
Holotype and paratype
Holotype (specimen N2, preparation PN8/17-2, number 673; illustrated in Nagovitsin et al., Reference Nagovitsin, Faizullin and Yakshin2004, pl. 2, fig. 4) and paratype (PN8/4-17/7-3; illustrated in Moczydłowska and Nagovitsin, Reference Moczydłowska and Nagovitsin2012, fig. 8E) are reposited in the Central Siberian Geological Museum of the United Institute of Geology, Geophysics, and Mineralogy, Siberian Branch of the Russian Academy of Sciences, Novosibirsk, Russia.
Occurrence
Ediacaran of East Siberia, South China, and northern India. East Siberia: Ura Formation of the Zhuya-Lena area (Nagovitsin et al., Reference Nagovitsin, Faizullin and Yakshin2004; Vorob'Eva et al., Reference Vorob'Eva, Sergeev and Chumakov2008; Golubkova et al., Reference Golubkova, Raevskaya and Kuznetsov2010; Sergeev et al., Reference Sergeev, Knoll and Vorob'Eva2011; Moczydłowska and Nagovitsin, Reference Moczydłowska and Nagovitsin2012). South China: Doushantuo Formation at Weng'an, Guizhou Province (Xiao et al., Reference Xiao, Zhou, Liu, Wang and Yuan2014) and Baizhu, Hubei Province (Yang et al., Reference Yang, Pang, Chen, Zhong and Yang2020). Northern India: Krol A Formation, Solan area (this paper).
Description and measurements
Medium-sized spheroidal vesicles with a moderate number of basally separate, digitate, hollow, and cylindrical to clavate processes that open to vesicle interior. Vesicle diameter up to 144 μm (Fig. 27.3), ~5 processes per 100 μm of vesicle periphery, process length at least 19–22 μm (~19% of vesicle diameter), process width 13–17 μm, and process spacing ~9 μm at base.
Materials
Two specimens illustrated in Figure 27.
Remarks
The specimens available are poorly preserved. In particular, the full length of the processes is not captured in thin sections. Most processes are captured in transverse or oblique sections (Fig. 27.2, 27.6, 27.8), making them appear to be circular, elliptical, or conical (Fig. 27.1, 27.2, lower center of Fig. 27.3), but the axially cut processes are cylindrical (Fig. 27.5) or clavate (Fig. 27.7). Overall, the process size, morphology, and density, as well as the vesicle size, of the Krol A specimens match the diagnosis of Tanarium digitiforme. Alternatively, these specimens could be assigned to Papillomembrana boletiformis Xiao et al., Reference Xiao, Zhou, Liu, Wang and Yuan2014, a taxon characterized by cylindrical processes with a bulbous or clavate termination. As noted above, Papillomembrana and the broadly defined Tanarium may be synonymous, and Xiao et al. (Reference Xiao, Zhou, Liu, Wang and Yuan2014) commented that some Doushantuo specimens of Papillomembrana compta Spjeldnaes, Reference Spjeldnaes1963, are better assigned to T. digitiforme. Indeed, P. compta has been recorded previously from the Infra-Krol Formation in the Nainital area of the Lesser Himalaya (Table 1). The Krol A specimens illustrated here, however, have relatively longer processes (~19% of vesicle diameter) than P. compta (6–8% of vesicle diameter) and P. boletiformis (~5% of vesicle diameter). In light of Grey's (Reference Grey2005) attempt to differentiate Tanarium from other acanthomorphs on the basis of process length, we choose to place the Krol A specimens under T. digitiforme.
Genus Weissiella Vorob'Eva, Sergeev, and Knoll, Reference Vorob'Eva, Sergeev and Knoll2009
Type species
Weissiella grandistella Vorob'Eva, Sergeev, and Knoll, Reference Vorob'Eva, Sergeev and Knoll2009.
Other species
Weissiella brevis Xiao et al., Reference Xiao, Zhou, Liu, Wang and Yuan2014, emend. Ouyang et al., Reference Ouyang, Zhou, Xiao, Guan, Chen, Yuan and Sun2021.
Weissiella brevis Xiao et al., Reference Xiao, Zhou, Liu, Wang and Yuan2014, emend. Ouyang et al., Reference Ouyang, Zhou, Xiao, Guan, Chen, Yuan and Sun2021
Figure 28
- Reference Xiao, Zhou, Liu, Wang and Yuan2014
Weissiella brevis Xiao et al., p. 61, fig. 38.
- Reference Shukla and Tiwari2014
Weissiella cf. grandistella; Shukla and Tiwari, p. 219, fig. 8A–E.
- Reference Ouyang, Zhou, Guan and Wang2015
Weissiella cf. brevis; Ouyang et al., p. 221, pl. III, figs. 7–13.
- Reference Ouyang, Zhou, Guan and Wang2015
Weissiella sp.; Ouyang et al., p. 221, pl. IV, figs. 8–13.
- Reference Ye, Tong, An, Tian, Zhao and Zhu2015
Weissiella sp.; Ye et al., p. 50, pl. I, figs. 15–19.
- Reference Sharma, Tiwari, Ahmad, Shukla, Shukla, Singh, Pandey, Ansari, Shukla and Kumar2016
Weissiella cf. grandistella; Sharma et al., fig. 4K (same as Shukla and Tiwari, Reference Shukla and Tiwari2014, fig. 8A).
- Reference Ouyang, Zhou, Xiao, Chen and Shao2019
Weissiella sp.; Ouyang et al., fig. 10E–H.
- Reference Liu and Moczydłowska2019
Weissiella grandistella; emend. Liu and Moczydłowska, p. 163, fig. 91A–E (part).
- Reference Shang, Liu and Moczydłowska2019
Weissiella grandistella; Shang et al., p. 28, fig. 19A, B.
- Reference Tian, Song, Ye, Hu, An, Zhao, Bottjer and Tong2020
Weissiella sp.; Tian et al., fig. 9K, L.
- Reference Liu, Qi, Fan, Guo, Pei, Huang, Cheng, Bian, Liu, Zhao and Zhang2021
Weissiella grandistella; Liu et al., fig. 5.3, 5.5.
- Reference Ouyang, Zhou, Xiao, Guan, Chen, Yuan and Sun2021
Weissiella brevis; emend. Ouyang et al., p. 40, figs. 6C, D, 23A–N.

Figure 28. Weissiella brevis. (1–4) DH-14-68.0-C-2, 23.8 × 112.6, EF-D12-4, VPIGM-4868, white and black arrows in (1) mark areas shown in (2) and (3, 4) (at two different focal levels). Black arrows in (2–4) mark cross-walls within processes. Note that arrows in (2, 3) are placed outside processes, and arrow in (4) is inside the process.
Holotype
VPIGM-4641 (WPB-3-4-4, 16.53132.6), reposited in the Museum of Geosciences at Virginia Polytechnic Institute, from unit 4A (probably equivalent to member II) of the Doushantuo Formation at Weng'an, Guizhou Province, South China (Xiao et al., Reference Xiao, Zhou, Liu, Wang and Yuan2014, fig. 38.1).
Occurrence
Ediacaran of South China and northern India. South China: unit 4A (probably equivalent to member II) of Doushantuo Formation, Weng'an, Guizhou Province (Xiao et al., Reference Xiao, Zhou, Liu, Wang and Yuan2014); member II and equivalent strata of Doushantuo Formation at Zhangcunping (Ye et al., Reference Ye, Tong, An, Tian, Zhao and Zhu2015; Ouyang et al., Reference Ouyang, Zhou, Xiao, Chen and Shao2019; Tian et al., Reference Tian, Song, Ye, Hu, An, Zhao, Bottjer and Tong2020), Jiulongwan (Ouyang et al., Reference Ouyang, Zhou, Xiao, Guan, Chen, Yuan and Sun2021), Jinguadun (Ouyang et al., Reference Ouyang, Zhou, Guan and Wang2015, Reference Ouyang, Zhou, Xiao, Guan, Chen, Yuan and Sun2021), Wuzhishan (Ouyang et al., Reference Ouyang, Zhou, Xiao, Guan, Chen, Yuan and Sun2021), Xiaofenghe (Liu and Moczydłowska, Reference Liu and Moczydłowska2019), and Changyang (Liu et al., Reference Liu, Qi, Fan, Guo, Pei, Huang, Cheng, Bian, Liu, Zhao and Zhang2021), Hubei Province; Doushantuo Formation at Liujing, Guizhou Province (Shang et al., Reference Shang, Liu and Moczydłowska2019). Northern India: Krol A Formation, Solan area (Shukla and Tiwari, Reference Shukla and Tiwari2014; Sharma et al., Reference Sharma, Tiwari, Ahmad, Shukla, Shukla, Singh, Pandey, Ansari, Shukla and Kumar2016; this paper).
Description and measurements
Medium-sized spheroidal vesicle with numerous evenly distributed processes that open to vesicle interior. Processes are relatively short, slightly taper toward an often-truncated distal end. Process interior is subdivided by transverse cross-walls. Vesicle diameter ~160 μm (Fig. 28.1), ~6–7 processes per 100 μm of vesicle periphery, process length ~15 μm (~10% of vesicle diameter), process width ~15 μm at base, and process spacing ~3 μm at base. One or more cross-walls present in each process, with a spacing of ~6 μm between cross-walls.
Materials
One specimen illustrated in Figure 28.
Remarks
Liu and Moczydłowska (Reference Liu and Moczydłowska2019) proposed that Weissiella brevis be synonymized with W. grandistella. However, W. brevis has distinctly smaller vesicles and more numerous processes than W. grandistella. Importantly, W. brevis has proportionally smaller and shorter processes relative to its vesicle size. With more materials available, the distinction between these two species has become clearer, and we follow Ouyang et al. (Reference Ouyang, Zhou, Xiao, Guan, Chen, Yuan and Sun2021) in regarding these two species as separate taxa. The distinction is even more apparent if the truncated distal end of W. brevis processes is a result of taphonomic breakage, because more completely preserved processes of W. brevis are expanded both basally and terminally (Ouyang et al., Reference Ouyang, Zhou, Xiao, Guan, Chen, Yuan and Sun2021), which is substantively different from the conical and distally tapering processes of W. grandistella. Based on the distinction between W. brevis and W. grandistella, as outlined in Ouyang et al. (Reference Ouyang, Zhou, Xiao, Guan, Chen, Yuan and Sun2021), several specimens previously illustrated as W. grandistella (Liu and Moczydłowska, Reference Liu and Moczydłowska2019; Shang et al., Reference Shang, Liu and Moczydłowska2019; Liu et al., Reference Liu, Qi, Fan, Guo, Pei, Huang, Cheng, Bian, Liu, Zhao and Zhang2021), W. cf. W. grandistella (Shukla and Tiwari, Reference Shukla and Tiwari2014; Sharma et al., Reference Sharma, Tiwari, Ahmad, Shukla, Shukla, Singh, Pandey, Ansari, Shukla and Kumar2016), and Weissiella sp. (Ye et al., Reference Ye, Tong, An, Tian, Zhao and Zhu2015; Ouyang et al., Reference Ouyang, Zhou, Xiao, Chen and Shao2019; Tian et al., Reference Tian, Song, Ye, Hu, An, Zhao, Bottjer and Tong2020) are here considered as W. brevis.
Results
Summary of δ13C and δ18O data
δ13C values of −8‰ to −15‰ occur in the first 30 m of the Krol A silty dolostones directly overlying the Krol Sandstone (Fig. 3.2; Table 2). The δ13C values shift to positive at the uppermost part of Krol A, with highest values up to +5.5‰. Most of the δ13C values of Krol B are in the range of +3.3‰ to +4.2‰, with a few down to +0.3‰ and −1.6‰. The δ13C values of Krol C are mostly around +3.0‰, with a few lower values below +2.0‰ and the highest values up to +4.1‰ (Fig. 3.2). The δ18O values of Krol A are very stable, with an average around −4‰ (Fig. 3.2, 3.3). In contrast, δ18O values of Krol B and Krol C are variable between −6‰ and −11‰ and they do not show a co-variation with δ13C values (Fig. 3.2, 3.3).
Table 2. Sample number, stratigraphic height, lithology, and δ13C and δ13O data from the Krol A to Krol C formation at sections DH-14 and DH2-14. δ13C and δ13O data are plotted in Figure 3.

Summary of Krol A microfossils: taxonomic treatment and stratigraphic distribution
We recovered 274 specimens of ornamented acritarchs, including 241 acanthomorph specimens belonging to 13 species and numerous specimens of the herkomorph species Dictyotidium grazhdankinii Xiao n. sp. (Table 3). The acanthomorphs can be divided into four groups based on their ornamentation, particularly size and shape of processes (Fig. 29; Table 4). The first and most common group of taxa—including Appendisphaera clava, A. tenuis, Cymatiosphaeroides forabilatus, and Mengeosphaera gracilis—is characterized by thin (<5 μm wide) and short processes (<15 μm and typically <10 μm long, or <5% of vesicle diameter), and accounts for 49% of ornamented acritarch specimens. The second group—including Appendisphaera grandis, A.? hemisphaerica, A. longispina, and A. setosa—accounts for 24% in abundance and is characterized by thin (<5 μm wide) and long processes (>10 μm and typically 15–30 μm long, or 5–10% of vesicle diameter). The third group, accounting for <2% in abundance, includes Tanarium digitiforme, T. cf. T. conoideum, and Weissiella brevis, which have thick processes (typically 10–15 μm wide) with variable lengths (typically 15–60 μm long, or 10–20% of vesicle diameter). The fourth group, representing 1% of the abundance, includes Asterocapsoides sinensis and Cavaspina tiwariae Xiao n. sp., which are characterized by obtusely conical processes. Herkomorphs are represented by one species, Dictyotidium grazhdankinii Xiao n. sp., whose abundance is likely overestimated because some heavily degraded leiospheres may be misidentified as this species.

Figure 29. Pie diagram showing relative abundance of acanthomorphic and herkomorphic taxa from the Krol A Formation. The four acanthomorph groups, as discussed in the text, are color coded (blue, thin and short processes; brown, thin and long processes; green, large and thick processes; red, obtuse processes) to show that the assemblage is dominated by acanthomorphs with thin processes. Schematic diagrams denoting the vesicle size and ornament morphology are shown. Note that scales are different for vesicles and ornaments (processes in acanthomorphs and reticula in herkomorphs).
Table 3. Summary of occurrence and abundance data for acanthomorphs, herkomorphs, and selected sphaeromorphs from the Krol A Formation. Number of specimens from each fossiliferous horizon is reported, with empty cells representing absence. A* = acanthomorphs; H* = herkomorphs; S* = sphaeromorphs. The abundance of Dictyotidium grazhdankinii Xiao n. sp. is probably an overestimate because poorly preserved sphaeromorphs may be mistakenly identified as this species. The abundance of unidentifiable acanthomorphs is likely underestimated because poorly preserved specimens may not be counted or photographed.

Table 4. Summary of measurements of acanthomorphs, herkomorphs, and selected sphaeromorphs from the Krol A Formation.

Taxonomic identification among species in the first two groups of acanthomorphs can be challenging, particularly when specimens are poorly preserved. Among the four species that have thin and short processes, Cymatiosphaeroides forabilatus is differentiated from the other species by its outer membrane, Mengeosphaera gracilis by its proportionally larger basal expansion relative to the apical spine, Appendisphaera clava by its small basal expansion and relatively long apical spine, and A. tenuis by its minute basal expansion or lack thereof. However, organic degradation and crystal growth can produce artifacts that resemble a basal expansion, taphonomic accumulation of organic material at the tip of processes with a uniform length can mimic an outer membrane, or the diaphanous outer membrane may be lost during diagenesis. In these cases, taxonomic identification relies on the consistency of morphological features (e.g., whether an outer membrane is continuous around the vesicle, whether a basal expansion is consistently present in most processes, and whether processes consistently penetrate the outer membrane, as in C. forabilatus). Still, many specimens have to be classified as unidentifiable (Table 3, Fig. 29, and many more that were not counted because of their poor preservation).
Similarly, among the four acanthomorph species with thin and long processes, Appendisphaera? hemisphaerica is unique in having clearly biform processes, a feature that belies its placement in the genus Appendisphaera (Liu et al., Reference Liu, Xiao, Yin, Chen, Zhou and Li2014a). The processes in A. grandis and A. longispina can have a basal expansion, but an inflection point is not apparent, thus these are not technically considered biform (see Fig. 24). The processes in A. setosa are largely cylindrical, without a basal expansion. The taxonomic identification of the rest of Krol A acanthomorphs—including Tanarium digitiforme, T. cf. T. conoideum, Weissiella brevis, Asterocapsoides sinensis, and Cavaspina tiwariae Xiao—is relatively straightforward, and their diagnostic features are schematically illustrated in Figure 29.
In addition to ornamented acritarchs, there are a number of sphaeromorphs in the Krol samples (several examples are illustrated in Fig. 30). Of importance are Osculosphaera arcelliformis Liu et al., Reference Liu, Xiao, Yin, Chen, Zhou and Li2014 (Fig. 30.4) and Schizofusa zangwenlongii Grey, Reference Grey2005 (Fig. 30.5, 30.6). The former has been known previously from the Tonian Svanbergfjellet Formation in Svalbard (Butterfield et al., Reference Butterfield, Knoll and Swett1994) and the upper Doushantuo Formation (member III) at Wangfenggang, Xiaofenghe, and Niuping sections in the Yangtze Gorge area (Liu et al., Reference Liu, Xiao, Yin, Chen, Zhou and Li2014a). The latter species is an eponymous species of the second acritarch biozone (i.e., the Tanarium tuberosum-Schizofusa zangwenlongii Assemblage Zone) in the lower Doushantuo Formation of the Yangtze Gorges area (Liu and Moczydłowska, Reference Liu and Moczydłowska2019).

Figure 30. Representative sphaeromorphs. (1) Leiosphaeridia tenuissima Eisenack, Reference Eisenack1958, S4-4-F2-7, 13.0 × 137.1, EF-P37-3, VPIGM-4892; (2) Leiosphaeridia jacutica (Timofeev, Reference Timofeev1966) Mikhailova and Jankauskas in Jankauskas et al., Reference Jankauskas, Mikhailova and Hermann1989, S4-4-F2-7, 7.3 × 136.1, EF-V36-1, VPIGM-4893; (3) Leiosphaeridia crassa (Naumova, Reference Naumova1949) Jankauskas in Jankauskas et al., Reference Jankauskas, Mikhailova and Hermann1989, DH-14-65.0-D-2, 15.2 × 127.8, EF-N27-2, VPIGM-4838; (4) Osculosphaera arcelliformis Liu et al., Reference Liu, Xiao, Yin, Chen, Zhou and Li2014, DH-14-64.1-C, 13.5 × 137.2, EF-O37-1/3, VPIGM- 4829; (5, 6) Schizofusa zangwenlongii Grey, Reference Grey2005, DH-14-68.0-A-2, 15.2 × 130.8, EF-N30-4, VPIGM-4861, same specimen at different focal levels, showing asymmetrical split and elongate folds.
To highlight their biostratigraphic significance of Krol A acritarchs, the stratigraphic occurrence and abundance of selected taxa—including all acanthomorphs, the herkomorph species Dictyotidium grazhdankinii Xiao n. sp., and the sphaeromorph species Schizofusa zangwenlongii—are presented in Table 3 and plotted in Figure 3 along with δ13C and δ18O data.
Like many other Ediacaran chert nodules, the Krol A samples contain abundant coccoidal and filamentous taxa that are traditionally regarded as cyanobacteria. Filamentous microfossils of the form genus Siphonophycus Schopf, Reference Schopf1968, are the most abundant fossils in the Krol A, many of which are preserved in rip-up fragments of microbial mats (Fig. 31.1–31.4). Another common form is Salome hubeiensis Zhang, Reference Zhang1986 (Fig. 31.5, 31.6), which was first reported from, and is widely present in, the Doushantuo Formation (e.g., Zhang et al., Reference Zhang, Yin, Xiao and Knoll1998; Xiao, Reference Xiao2004b). Other filamentous forms include Botominella lineata Reitlinger, Reference Reitlinger1959 (Fig. 32.1, 32.2), Obruchevella sp. (Fig. 32.3, 32.4), Oscillatoriopsis breviconvexa Schopf and Blacic, Reference Schopf and Blacic1971 (Fig. 32.5–32.7), and Polytrichoides lineatus Hermann, Reference Hermann and Timofeev1974 (Fig. 32.8, 32.9). These filamentous forms are common, but they have very long stratigraphic ranges and thus have limited biostratigraphic significance.

Figure 31. Filamentous microfossils. (1, 2) Fragments of microbial mat consisting of entangled sheaths of Siphonophycus spp., DH-14-65.0-B-2, 18.5 × 117.5, EF-J17, VPIGM-4836, rectangle in (1) marks area magnified in (2); (3, 4) fragment of microbial mat with Siphonophycus filaments, some of which are partially pyritized, S4-4-F2-5-A, 15.1 × 121.0, EF-N21-1, VPIGM-4903, arrow in (3) marks area magnified in (4); (5, 6) Salome hubeiensis Zhang, Reference Zhang1986; (5), S4-4-F2-7-A, 21.1 × 132.2, EF-F32-3, VPIGM-4905; (6), S4-4-F2-14-A, 9.6 × 129.9, EF-S30-1, VPIGM-4909.

Figure 32. Filamentous microfossils. (1, 2) Carbonized filaments identified by Sharma et al. (Reference Sharma, Shukla and Sergeev2021) as Botominella lineata Reitlinger, Reference Reitlinger1959; (1), DH-14-65.0-B, 18.6 × 107.0, EF-J7-3, VPIGM-4835; (2), DH-14-68.0-A-2, 4.5 × 116.2, EF-Y16-1, VPIGM-4862; (3) Obruchevella sp., S4-4-F2-5, 12.5 × 109.5, EF-Q9-2, VPIGM-4877; (4) Obruchevella sp. (arrow) and Siphonophycus spp., DH-14-68.0-B-2, 14.5 × 118.5, EF-O18-2, VPIGM-4866; (5–7) Oscillatoriopsis breviconvexa Schopf and Blacic, Reference Schopf and Blacic1971; (5, 6), S4-4-F2-7, 11.1 × 110.8, EF-R11-3, VPIGM-4891, rectangle in (5) marks area magnified in (6); (7), DH-14-68.0-A, 12.0 × 131.2, EF-Q31-1, VPIGM-4860; (8, 9) Polytrichoides lineatus Hermann, Reference Hermann and Timofeev1974, S4-4-F2-5, 8.9 × 129.3, EF-T29-4, VPIGM-4880, same specimen at different levels, showing bundled filaments.
Finally, confirming previous reports (e.g., Shukla et al., Reference Shukla, Mathur, Babu and Srivastava2008), we have identified a number of multicellular algae from the Krol A chert nodules. Identification of three-dimensionally silicified multicellular algae in thin sections is a challenge (Zhang et al., Reference Zhang, Yin, Xiao and Knoll1998; Xiao et al., Reference Xiao, Knoll, Yuan and Pueschel2004). Nonetheless, several taxa are recognizable on the basis of their cell arrangement patterns. For example, closely arranged and nested cell packets are identified as Sarcinophycus radiatus Xiao and Knoll, Reference Xiao and Knoll1999 (Fig. 33.1), spherical thalli with compact parenchymatous cells are regarded as Wengania minuta Xiao, Reference Xiao2004 (Fig. 33.2) and W. exquisita Zhang et al., Reference Zhang, Yin, Xiao and Knoll1998 (Fig. 33.5–33.6), and pseudoparenchymatous thalli consisting of linearly aligned cells but without a well-defined cortex-medulla differentiation are identified as Thallophycoides phloeatus Zhang and Yuan, Reference Zhang and Yuan1992 (Fig. 33.3, 33.4). These taxa have been reported previously from the Doushantuo Formation in South China (Zhang, Reference Zhang1989; Zhang and Yuan, Reference Zhang and Yuan1992; Zhang et al., Reference Zhang, Yin, Xiao and Knoll1998; Xiao, Reference Xiao2004b; Xiao et al., Reference Xiao, Knoll, Yuan and Pueschel2004; Liu et al., Reference Liu, Xiao, Yin, Chen, Zhou and Li2014a; Shang et al., Reference Shang, Liu and Moczydłowska2019; Ouyang et al., Reference Ouyang, Zhou, Xiao, Guan, Chen, Yuan and Sun2021). It is worth mentioning that W. exquisita also has been reported from phosphorite of the Ediacaran Birmania Formation in Rajasthan of western India (Hughes et al., Reference Hughes, Myrow, McKenzie, Xiao, Banerjee, Stockli and Tang2015), and multicellular algal fossils have been reported previously from the Infra-Krol Formation (Tiwari and Pant, Reference Tiwari and Pant2004) and the approximately equivalent Chambaghat Formation in the western Krol Belt (Shukla et al., Reference Shukla, Babu, Mathur and Srivastava2005a), as well as from the Krol C Formation in the Garhwal syncline of the Krol Belt (Singh and Rai, Reference Singh and Rai2013). Currently available data seem to indicate that, although these multicellular algal taxa have important implications for the evolution of multicellularity, they have long stratigraphic ranges and thus are not useful in refining Ediacaran biostratigraphy.

Figure 33. Multicellular algae. (1) Sarcinophycus radiatus Xiao and Knoll, Reference Xiao and Knoll1999, DH-14-52.6-B, 13.6 × 120.5, EF-O20-2, VPIGM-4828; (2) Wengania minuta Xiao, Reference Xiao2004, S4-4-F2-13-A, 11.8 × 127.0, EF-Q26-4, VPIGM-4908; (3, 4) Thallophycoides phloeatus Zhang and Yuan, Reference Zhang and Yuan1992, DH-14-68.0-A-2, 7.3 × 112.0, EF-V12, VPIGM-4863, arrow in (3) marks area magnified in (4); (5, 6) Wengania exquisita Zhang et al., Reference Zhang, Yin, Xiao and Knoll1998, S4-4-F2-5, 24.1 × 102.7, EF-D3-3, VPIGM-4879, rectangle in (5) marks area magnified in (6).
Discussion
Chemostratigraphic correlation
The overall δ13C and δ18O values of the Krol A–Krol C interval are within the range of isotope values of early–middle Ediacaran strata at other sections of the Krol Belt (Kaufman et al., Reference Kaufman, Jiang, Christie-Blick, Banerjee and Rai2006). However, the negative δ13C excursion at the Krol B-C transition from other sections (Kaufman et al., Reference Kaufman, Jiang, Christie-Blick, Banerjee and Rai2006) is not well displayed in the section analyzed in this study. The potential cause could be that the upper part of Krol B Formation is truncated in the current section, as evinced by the presence of a sandstone layer at the top of the Krol B Formation. The negative δ13C excursion from the lower Krol A was not documented in previous studies due to the lack of exposure, although a few negative δ13C values at the transition from Krol Sandstone to the Krol A Formation and its correlative interval were reported in Kaufman et al. (Reference Kaufman, Jiang, Christie-Blick, Banerjee and Rai2006). Negative δ13C values of the lower Krol A correspond with δ18O values consistently around −4‰ (Fig. 3.3), implying either that the δ18O values have not been significantly modified by diagenesis or that diagenesis may have uniformly reset the δ18O values to −4‰. The latter is more likely, considering the more variable δ18O values of Krol B and Krol C in the same section and the very low δ13C values down to −15‰ in the lower Krol A. Therefore, even though we consider that the negative shift in δ13C in the lower Krol A may represent a real chemostratigraphic excursion, the magnitude of this excursion may have been exaggerated by diagenetic alteration.
In combination with previously published isotope data from the Krol Belt (Kaufman et al., Reference Kaufman, Jiang, Christie-Blick, Banerjee and Rai2006; Etienne et al., Reference Etienne, Allen, Guerroue, Heaman, Ghosh, Islam, Arnaud, Halverson and Shields-Zhou2011), we construct a composite δ13C curve for the Ediacaran strata (Infra-Krol–Krol C) of the Krol Belt and propose a correlation with the δ13C record of the Doushantuo and Dengying formations in the Yangtze Platform (Fig. 34). Accepting Kaufman et al.'s (Reference Kaufman, Jiang, Christie-Blick, Banerjee and Rai2006) correlation of the negative δ13C excursions at Krol B-C and upper Doushantuo Formation (EN3, which is widely regarded as equivalent to the Shuram negative δ13C excursion, Jiang et al., Reference Jiang, Kaufman, Christie-Blick, Zhang and Wu2007; McFadden et al., Reference McFadden, Huang, Chu, Jiang, Kaufman, Zhou, Yuan and Xiao2008), the negative δ13C excursion in the lower Krol A is most parsimoniously correlated with the negative δ13C excursion EN2 in the uppermost member II of the Doushantuo Formation in the Yangtze Gorges area in South China (Fig. 34). This chemostratigraphic correlation can illuminate and be further tested by biostratigraphic correlation.

Figure 34. Integrated δ13C chemostratigraphic and acritarch biostratigraphic correlation between Lesser Himalaya (northern India) and Yangtze Gorges area (South China). The δ13C curve of Lesser Himalaya is summarized from Kaufman et al. (Reference Kaufman, Jiang, Christie-Blick, Banerjee and Rai2006) and Etienne et al. (Reference Etienne, Allen, Guerroue, Heaman, Ghosh, Islam, Arnaud, Halverson and Shields-Zhou2011; cap dolostone data), supplemented by new data from this study (Fig. 3.2; Table 2). Note that the thickness of the Infra-Krol Formation is not fully drawn because no carbonate δ13C data are available (dashed curve). The δ13C curve of the Yangtze Gorges area is summarized from Jiang et al. (Reference Jiang, Kaufman, Christie-Blick, Zhang and Wu2007) and McFadden et al. (Reference McFadden, Huang, Chu, Jiang, Kaufman, Zhou, Yuan and Xiao2008). Zircon U-Pb ages of the Doushantuo Formation are from Condon et al. (Reference Condon, Zhu, Bowring, Wang, Yang and Jin2005). Black and red arrows mark approximate stratigraphic horizons of, respectively, Krol A microfossils reported in this paper and the occurrence of Tianzhushania spinosa and T. polysiphonia in the Infra-Krol Formation reported by Joshi and Tiwari (Reference Joshi and Tiwari2016). Solid vertical lines show approximate stratigraphic range of selected acritarch taxa in the Doushantuo Formation (see occurrence information in Systematic Paleontology; Liu et al., Reference Liu, Xiao, Yin, Chen, Zhou and Li2014a; Liu and Moczydłowska, Reference Liu and Moczydłowska2019). It is uncertain whether acanthomorphs from Liujing in Guizhou Province (Shang et al., Reference Shang, Liu and Moczydłowska2019) belong to member II (based on biostratigraphic correlation advocated in this paper) or upper member III to member IV of the Doushantuo Formation (based on lithostratigraphic correlation); the latter scenario is represented in the dashed vertical lines. Important zonal taxa are color coded according to the four acritarch assemblage zones recognized in the Yangtze Gorges area of South China (Liu and Moczydłowska, Reference Liu and Moczydłowska2019): (a) Appendisphaera grandis-Weissiella grandistella-Tianzhushania spinosa Assemblage Zone; (b) Tanarium tuberosum-Schizofusa zangwenlongii Assemblage Zone; (c) Tanarium conoideum-Cavaspina basiconica Assemblage Zone; (d) Tanarium pycnacanthum-Ceratosphaeridium glaberosum Assemblage Zone. Note that all Krol A acritarch species, except new and open-nomenclature taxa, are also present in the Doushantuo Formation. Light yellow band, which includes the dark yellow band, represents permissive correlation between the fossiliferous lower Krol A Formation and the upper member II of the Doushantuo Formation in the Yangtze Gorges area based on biostratigraphic data. Dark yellow band denotes preferred correlation based on integrative chemo- and biostratigraphic data. See text for details.
Biostratigraphic correlation
In this section, we consider possible biostratigraphic correlation between the Krol Group in the Lesser Himalaya and the Doushantuo Formation in the Yangtze Gorges area. Biostratigraphic investigations of the early–middle Doushantuo Formation in the Yangtze Gorges area in the past four decades (e.g., Yin and Li, Reference Yin and Li1978; Zhang et al., Reference Zhang, Yin, Xiao and Knoll1998; Zhou et al., Reference Zhou, Xie, McFadden, Xiao and Yuan2007; McFadden et al., Reference McFadden, Xiao, Zhou and Kowalewski2009; Yin et al., Reference Yin, Liu, Chen, Tang, Gao and Wang2009; Xiao et al., Reference Xiao, McFadden, Peek, Kaufman, Zhou, Jiang and Hu2012; Liu et al., Reference Liu, Yin, Chen, Tang and Gao2013, Reference Liu, Xiao, Yin, Chen, Zhou and Li2014a, Reference Liu, Chen, Zhu, Li, Yin and Shangb; Liu and Moczydłowska, Reference Liu and Moczydłowska2019; Ouyang et al., Reference Ouyang, Zhou, Xiao, Guan, Chen, Yuan and Sun2021) have established a foundation for acritarch-based biostratigraphy. Earlier studies of Doushantuo acritarchs led to the recognition of two biozones separated by the negative δ13C excursion EN2 (McFadden et al., Reference McFadden, Huang, Chu, Jiang, Kaufman, Zhou, Yuan and Xiao2008, Reference McFadden, Xiao, Zhou and Kowalewski2009; Yin et al., Reference Yin, Liu, Chen, Tang, Gao and Wang2009; Liu et al., Reference Liu, Xiao, Yin, Chen, Zhou and Li2014a; Xiao et al., Reference Xiao, Zhou, Liu, Wang and Yuan2014). These two biozones (the Tianzhushania spinosa biozone in member II and the Tanarium conoideum-Hocosphaeridium scaberfacium-H. anozos biozone in member III of the Doushantuo Formation) were vaguely understood as acme biozones characterized by the abundant occurrence of the eponymous species (Xiao et al., Reference Xiao, Zhou, Liu, Wang and Yuan2014). However, Liu et al. (Reference Liu, Xiao, Yin, Chen, Zhou and Li2014a) indicated that the lower boundaries of these two biozones could be defined by the first occurrence of T. spinosa in member II and H. anozos in member III, respectively. Subsequent investigation revealed that the four eponymous species have overlapping stratigraphic ranges; for example, T. conoideum and H. anozos are found co-occurring with T. spinosa in the Doushantuo Formation at Weng'an in South China (Xiao et al., Reference Xiao, Zhou, Liu, Wang and Yuan2014), and both H. scaberfacium Zang in Zang and Walter, Reference Zang and Walter1992, and H. anozos (Willman in Willman and Moczydłowska, Reference Willman and Moczydłowska2008) Xiao et al., Reference Xiao, Zhou, Liu, Wang and Yuan2014, extend down-section to member II of the Doushantuo Formation (Hawkins et al., Reference Hawkins, Xiao, Jiang, Wang and Shi2017; Liu and Moczydłowska, Reference Liu and Moczydłowska2019; Liu et al., Reference Liu, Qi, Fan, Guo, Pei, Huang, Cheng, Bian, Liu, Zhao and Zhang2021). Thus, the two biozones cannot be used in the sense of range biozone, and the concept of acme biozone is difficult to apply here because abundance data are not always available and also because of arbitrary nature of defining an acme biozone. The problem is further complicated by the general lack of acritarchs at the member II-III transition (barren interval) that hosts the negative δ13C excursion EN2 and separates the two vaguely defined acme biozones.
In a recent attempt to clarify the acritarch biostratigraphy of the Doushantuo Formation, Liu and Moczydłowska (Reference Liu and Moczydłowska2019) proposed four assemblage zones, in ascending order: (a) Appendisphaera grandis-Weissiella grandistella-Tianzhushania spinosa Assemblage Zone, (b) Tanarium tuberosum-Schizofusa zangwenlongii Assemblage Zone, (c) Tanarium conoideum-Cavaspina basiconica Assemblage Zone, and (d) Tanarium pycnacanthum-Ceratosphaeridium glaberosum Assemblage Zone. The lower boundary of each assemblage zone is defined by the first joint appearance of the eponymous species (plural), although the upper boundary of the fourth assemblage zone has not been defined. The first three assemblage zones occur in member II of the Doushantuo Formation, and together they are roughly equivalent to the Tianzhushania spinosa biozones recognized by earlier authors. The fourth assemblage zone, which is separated from the third assemblage zone by EN2 and a barren interval, occurs in member III of the Doushantuo Formation and is roughly equivalent to the T. conoideum-H. scaberfacium-H. anozos biozone of previous authors.
One challenge in the application of these assemblage zones is that several eponymous species appear to have very long stratigraphic ranges. For example, if the Semri Group of the lower Vindhyan Supergroup in the Chambal Valley of eastern Rajasthan (India) is proven to be Paleo-/Mesoproterozoic (see Hughes, Reference Hughes2017, for further discussion), then at least five of the nine eponymous species (Appendisphaera grandis; Cavaspina basiconica; Ceratosphaeridium glaberosum Grey, Reference Grey2005; Tanarium conoideum; and T. tuberosum) and the Tanarium conoideum-Cavaspina basiconica Assemblage Zone of Liu and Moczydłowska (Reference Liu and Moczydłowska2019) would extend to the Paleo-/Mesoproterozoic because these species have all been reported from the Semri Group (Prasad and Asher, Reference Prasad and Asher2016). The same can also be said of several eponymous species (Ceratosphaeridium glaberosum; Gyalosphaeridium pulchrum Zang in Zang and Walter, Reference Zang and Walter1992; Schizofusa risoria Grey, Reference Grey2005; and Tanarium conoideum) of the Australian acanthomorph assemblage zones established by Grey (Reference Grey2005). This problem highlights the importance of an independent assessment of the depositional age of the Semri Group in the Chambal Valley, as well as a critical re-examination of the Semri acanthomorphs. Additionally, the long stratigraphic ranges of certain eponymous taxa (e.g., Appendisphaera grandis, Cavaspina basiconica, Ceratosphaeridium glaberosum, and Tanarium conoideum) is apparent because of their potential presence in upper Ediacaran–lower Cambrian strata (Ouyang et al., Reference Ouyang, Guan, Zhou and Xiao2017; Anderson et al., Reference Anderson, McMahon, Macdonald, Jones and Briggs2019; Grazhdankin et al., Reference Grazhdankin, Nagovitsin, Golubkova, Karlova, Kochnev, Rogov and Marusin2020) (see also Golubkova et al., Reference Golubkova, Zaitseva, Kuznetsov, Dovzhikova and Maslov2015, although Vorob'Eva et al., Reference Vorob'Eva, Sergeev and Knoll2009, assigned a middle Ediacaran age to the Keltma acanthomorph assemblage in the Timan Ridges of Baltica).
An additional challenge is related to the fact that the assemblage zones of Liu and Moczydłowska (Reference Liu and Moczydłowska2019) are each defined at the base by the joint first appearance of multiple eponymous species. If only one of the eponymous species is found and it happens to be a long-ranging taxon, it does not necessarily indicate a correlation with an assemblage zone bearing its name because this species can occur considerably below or above, particularly when the eponymous species (plural) that are used in combination to define the assemblage zone have drastically different first appearances. This problem can be exacerbated by stratigraphic condensation and cryptic unconformities, which may be the case in parts of the Doushantuo Formation (Liu and Moczydłowska, Reference Liu and Moczydłowska2019).
On the other hand, Ouyang et al. (Reference Ouyang, Zhou, Xiao, Guan, Chen, Yuan and Sun2021) have demonstrated that some eponymous species of Liu and Moczydłowska's (Reference Liu and Moczydłowska2019) assemblage zones do have consistent first appearance data in member II of the Doushantuo Formation in the Yangtze Gorges area. For example, Tianzhushania spinosa, Appendisphaera grandis, and Weissiella brevis tend to first appear near the base of member II of the Doushantuo Formation. Thus, barring the unresolved issue related to the age of the Semri Group, it is possible that the eponymous species chosen to define an assemblage zone may actually have first appearance data close to each other. If so, the presence of a single eponymous species is still useful biostratigraphic information, even if it may range up-section to a younger assemblage zone. In other words, the presence of a single eponymous species may be taken as a maximum age estimate as defined by the assemblage zone bearing its name. It is under this assumption that a biostratigraphic correlation between the Lesser Himalaya and the Yangtze Gorges area is made (Fig. 34).
The biostratigraphic correlation between the Lesser Himalaya and the Yangtze Gorges area is built on the common occurrence of numerous acritarch species, including several zonal taxa (Fig. 34). The presence of Tianzhushania spinosa and T. polysiphonia in the Infra-Krol Formation (Joshi and Tiwari, Reference Joshi and Tiwari2016) and the apparent absence of taxa indicative of the Tanarium tuberosum-Schizofusa zangwenlongii Assemblage Zone (Table 1), as well as the stratigraphic proximity between the Infra-Krol Formation and the basal Ediacaran cap dolostone, indicate a correlation with the Appendisphaera grandis-Weissiella grandistella-Tianzhushania spinosa Assemblage Zone in the Yangtze Gorges area. The presence of Schizofusa zangwenlongii in the Krol A Formation (this paper) invites a correlation with the Tanarium tuberosum-Schizofusa zangwenlongii Assemblage Zone or higher. Considering that Schizofusa zangwenlongii is actually more common in Doushantuo strata above the Tanarium tuberosum-Schizofusa zangwenlongii Assemblage Zone (Liu and Moczydłowska, Reference Liu and Moczydłowska2019), that Tanarium cf. T. conoideum from the Krol A Formation (Fig. 26) may actually be T. conoideum, and that the negative δ13C excursion in the lower Krol A Formation is correlated with EN2 in uppermost member II of the Doushantuo Formation, the Krol A assemblage is more likely correlated with the Tanarium conoideum-Cavaspina basiconica Assemblage Zone. Insofar as the Krol A assemblage is associated with the rising arm of a negative δ13C excursion that is equivalent to EN2, it also fills a gap represented by a barren interval in the Yangtze Gorges area (Liu and Moczydłowska, Reference Liu and Moczydłowska2019), where direct association of acanthomorphs and EN2 has not been documented due to the lack of fossiliferous chert nodules in this interval (McFadden et al., Reference McFadden, Xiao, Zhou and Kowalewski2009). This correlation predicts that the second and fourth assemblage zones (i.e., the Tanarium tuberosum-Schizofusa zangwenlongii and Tanarium pycnacanthum-Ceratosphaeridium glaberosum assemblage zones) should be recorded, respectively, below and above the chert nodule interval of the lower Krol A Formation sampled in this study. The presence of an unconformity at the base of the Krol Sandstone compromises our effort to test this prediction, but an exploration of the upper Krol A and Krol B formations is warranted to search for microfossils indicative of the Tanarium pycnacanthum-Ceratosphaeridium glaberosum Assemblage Zone.
Whereas the Krol A Formation in the Lesser Himalaya and the lower Doushantuo Formation in the Yangtze Gorges show notable similarity in acritarch presence data, which facilitates biostratigraphic correlation, we would also like to point out some perceived differences in taxonomic abundance. We note that the Krol A acritarch assemblage is characterized by the abundance of acanthomorphs with short and thin processes (e.g., Appendisphaera clava, A. tenuis, Cymatiosphaeroides forabilatus; Fig. 28), as well as the scarcity of acanthomorphs with large and long processes (e.g., various species of Tanarium, Weissiella, and Sinosphaera Zhang et al., Reference Zhang, Yin, Xiao and Knoll1998). As noted by Shukla and Tiwari (Reference Shukla and Tiwari2014, p. 215) the “absence of Tanarium, the marker acritarch taxon of the upper Doushantuo assemblage in the Krol Group, is very peculiar.” That the Krol A assemblage is likely correlated with one of the assemblage zones in the lower rather than the upper Doushantuo Formation may partially explain this peculiarity, but even the lower Doushantuo Formation (member II) in the Yangtze Gorges area contains abundant acanthomorphs with large and long processes (Liu and Moczydłowska, Reference Liu and Moczydłowska2019; Ouyang et al., Reference Ouyang, Zhou, Xiao, Guan, Chen, Yuan and Sun2021). Different sampling intensities between the two regions are unlikely to have preferentially biased against acanthomorphs with large and long processes relative to those with short and thin processes. For the same reason, taphonomic bias is an unlikely explanation for this difference either, considering that both the Krol A assemblage in the Solan area and the Doushantuo acritarchs in the Yangtze Gorges area are taphonomically similar.
Paleoenvironmental and paleogeographic factors also may have played important roles in dictating taxonomic similarities and differences among acanthomorph assemblages in South China and northern India. As commented earlier in the paper, the overall taxonomic similarities and our ability to correlate Ediacaran acanthomorph assemblages between South China and northern India is facilitated by their paleogeographic proximity. However, there are more nuances. To elaborate, it is instructive to compare and contrast the Krol A assemblage against Doushantuo acritarchs from Liujing and Weng'an in Guizhou Province. The Liujing and Weng'an assemblages are paleogeographically close (~100 km apart; Fig. 35), but taphonomically and environmentally different; Liujing fossils are silicified in chert nodules in shales and argillaceous dolostones, whereas Weng'an fossils are phosphatized in intraclastic phosphorites. Like the Krol A assemblage, the Liujing assemblage is numerically dominated by acanthomorphs with short and thin processes, particularly Cymatiosphaeroides forabilatus (accounting for 48.7% of acanthomorph abundance) and Mengeosphaera membranifera Shang et al., Reference Shang, Liu and Moczydłowska2019 (accounting for 21.3% of acanthomorph abundance), the latter species of which is similar to Mengeosphaera gracilis except the presence of an outer membrane. In contrast, qualitative data from Weng'an show that acanthomorphs with large processes (e.g., Mengeosphaera chadianensis) seem to be the most common taxa (Xiao et al., Reference Xiao, Zhou, Liu, Wang and Yuan2014). The zonal microfossils, including Appendisphaera grandis, Cavaspina basiconica, Schizofusa zangwenlongii, Tanarium conoideum, Tanarium tuberosum, and Weissiella grandistella, are present in the Liujing assemblage (Shang et al., Reference Shang, Liu and Moczydłowska2019), whereas Appendisphaera grandis, Cavaspina basiconica, Tanarium conoideum, Tanarium tuberosum, Tianzhushania spinosa, and Weissiella brevis have been found in Weng'an (Xiao et al., Reference Xiao, Zhou, Liu, Wang and Yuan2014). These fossils indicate that the Liujing and Weng'an assemblages are unlikely correlated with the Tanarium pycnacanthum-Ceratosphaeridium glaberosum Assemblage Zone in the Yangtze Gorges area, but are probably part of the Tanarium conoideum-Cavaspina basiconica Assemblage Zone. If so, then the Krol A, Liujing, and Weng'an assemblages are broadly correlated to the same assemblage zone.

Figure 35. Representative localities from Ediacaran basins where acanthomorphs have been reported. Also included are terminal Ediacaran to early Cambrian assemblages from the Lena-Anabar Basin (Grazhdankin et al., Reference Grazhdankin, Nagovitsin, Golubkova, Karlova, Kochnev, Rogov and Marusin2020) and the Khuvsgul Basin (Anderson et al., Reference Anderson, Macdonald, Jones, McMahon and Briggs2017, Reference Anderson, McMahon, Macdonald, Jones and Briggs2019). Ediacaran acanthomorphs were reported from the Zavkhan material (Ragozina et al., Reference Ragozina, Dorjnamjaa, Serezhnikova, Zaitseva and Enkhbaatar2016), but the published illustrations are not convincing. (1) Localities on a modern geographic map. The scarcity of Ediacaran acanthomorphs in the western hemisphere is likely due to poor sampling intensity. (2) Localities on a ca. 600 Ma paleogeographic map (Merdith et al., Reference Merdith, Williams, Collins, Tetley, Mulder, Blades, Young, Armistead, Cannon, Zahirovic and Müller2021). The paleogeographic location of Svalbard is uncertain, but it probably was close to Greenland (Gasser, Reference Gasser, Corfu, Gasser and Chew2013). Note that Ediacaran acanthomorphs are concentrated in low latitudes and the possibility of two paleobiogeographic provinces (Gondwana vs. Laurentia-Baltica-Siberia). Also note the paleogeographic proximity between Lesser Himalaya and South China (particularly Liujing and Weng'an). Maps were generated using the software gplate.
Thus, as revealed by the abundance data, the similarity between the Krol A and Liujing assemblages, as well as the difference between the Krol A and Weng'an assemblages, can be considered in a paleoenvironmental, taphonomic, and paleogeographic context. Whereas both Liujing and Weng'an are equally close to Krol A (Fig. 35), the former is additionally similar to the Krol A in taphonomy and paleoenvironment. We note that there is currently no independent chronostratigraphic data to constrain the age of the Liujing assemblage, so correlation of the Liujing assemblage with the Tanarium conoideum-Cavaspina basiconica Assemblage Zone awaits corroboration with additional data. Indeed, the fossiliferous units (beds 4 and 5) at Liujing are somewhat similar in lithostratigraphy to upper member III and member IV of the Doushantuo Formation in the Yangtze Gorges area (the reason for the dashed vertical lines in Fig. 34). Alternatively, the fossiliferous units at Liujing may be equivalent to member II of the Doushantuo Formation and strata equivalent to members III-IV may be missing at Liujing (as is the case in the eastern Huangling anticline of the Yangtze Gorges area, where upper member III and member IV are missing; Xiao et al., Reference Xiao, Bykova, Kovalick and Gill2017; Zhou et al., Reference Zhou, Xiao, Wang, Guan, Ouyang and Chen2017). If this is the case, then all dashed vertical lines in Figure 34 should be removed. A chemostratigraphic test of these two correlations requires δ13C data from the Liujing section, which are currently unavailable. Nonetheless, the general statement stands that paleogeography, paleoenvironments, and taphonomy should be considered when carrying out Ediacaran biostratigraphic correlation using acanthomorphs.
If, as discussed above, the Liujing and Krol assemblages are correlated to the Tanarium conoideum-Cavaspina basiconica Assemblage Zone, their similarity in taxonomic presence and abundance is not unexpected, given their paleogeographic location (Fig. 35). Nine of the 12 biostratigraphically significant species from Krol A are also present at Liujing (see dashed vertical lines in Fig. 34), in addition, Tanarium conoideum may be present in both assemblages (see taxonomic discussion in Tanarium cf. T. conoideum). More importantly, both assemblages are numerically dominated by acanthomorphs with thin processes (e.g., Appendisphaera grandis, A.? hemisphaerica, A. longispina, A. setosa, A. tenuis, Cymatiosphaeroides forabilatus, and Mengeosphaera gracilis). From a paleogeographic viewpoint, this similarity makes perfect sense. According to several paleogeographic reconstructions (Jiang et al., Reference Jiang, Sohl and Christie-Blick2003a; Qi et al., Reference Qi, Xu, Cawood and Du2018; Merdith et al., Reference Merdith, Williams, Collins, Tetley, Mulder, Blades, Young, Armistead, Cannon, Zahirovic and Müller2021), the Lesser Himalaya was either directly facing or immediately juxtaposing the southwestern side of the Yangtze block. In these paleogeographic configurations, the Liujing section was paleogeographically closer to the Lesser Himalaya than the Yangtze Gorges area was to the Lesser Himalaya during the Ediacaran Period (Fig. 35). Hence, even though the Krol assemblage is generally similar to lower Doushantuo acritarchs in South China, it is particularly similar to the Liujing assemblage in both presence and abundance data. This similarity is related to their biostratigraphic equivalence, taphonomic comparability, and paleogeographic proximity.
The integrated bio- and chemostratigraphic correlation between the Krol A and Doushantuo Formation illustrates the promise of Ediacaran acritarchs as important biostratigraphic tools for global correlation. The natural next step is to apply the same integrative approach to correlate Ediacaran strata in east Gondwana (South China, India, and South Australia) and beyond. With a solid chronostratigraphic framework, we can also begin to explore possible signs of paleobiogeographic differentiation of Ediacaran acanthomorphs. For example, according to the paleogeographic reconstruction of Merdith et al. (Reference Merdith, Williams, Collins, Tetley, Mulder, Blades, Young, Armistead, Cannon, Zahirovic and Müller2021), Ediacaran acanthomorphs seem to be concentrated in low paleolatitudes. It is also tempting to recognize a Tianzhushania paleobiogeographic province in part of east Gondwana, as indicated by the occurrence of this genus in northern India and South China (Liu et al., Reference Liu, Xiao, Yin, Chen, Zhou and Li2014a; Xiao et al., Reference Xiao, Zhou, Liu, Wang and Yuan2014; Joshi and Tiwari, Reference Joshi and Tiwari2016). As a side note, specimens published as ?Trachyhystrichosphaera sp. from the Scotia Group in Svalbard are somewhat similar to Tianzhushania polysiphonia in its clustered distribution of cylindrical processes, but do not seem to preserve other key features of the genus Tianzhushania (i.e., a multilaminate layer surrounding the vesicle wall and an outer membrane supported by the cylindrical processes), so their identification as T. polysiphonia remains to be confirmed. The speculation of a Tianzhushania paleobiogeographic province warrants further investigation with biostratigraphic, taphonomic, and paleoenvironmental controls. Nonetheless, we are confident that the growing data of Ediacaran acritarchs will soon illuminate a key component of Ediacaran paleobiogeography, which thus far has been derived mainly from macrofossils (Waggoner, Reference Waggoner1999; Boag et al., Reference Boag, Darroch and Laflamme2016).
Conclusions
This study offers an instructive example of inter-basinal correlation of early Ediacaran strata between the Lesser Himalaya and the Yangtze Gorges area using integrative bio- and chemostratigraphic data, and the results are encouraging. Based on the common occurrence of Tianzhushania spinosa and T. polysiphonia, the Infra-Krol Formation in the Lesser Himalaya is correlated with the Appendisphaera grandis-Weissiella grandistella-Tianzhushania spinosa Assemblage Zone of the lower Doushantuo Formation in the Yangtze Gorges area. The Krol A Formation in the Lesser Himalaya contains over a dozen acanthomorph species, including two new species—Cavaspina tiwariae Xiao n. sp. and Dictyotidium grazhdankinii Xiao n. sp.—as well as numerous sphaeromorphs, filaments, coccoids, and multicellular algae. Many of these fossils, including all but the new and open-nomenclature acanthomorph taxa, are also present in the Doushantuo Formation. These microfossils indicate a biostratigraphic correlation with the Tanarium tuberosum-Schizofusa zangwenlongii Assemblage Zone or Tanarium conoideum-Cavaspina basiconica Assemblage Zone of the lower Doushantuo Formation in the Yangtze Gorges area. The prominent negative δ13C excursion in association with the Krol A microfossils is correlated with the negative δ13C excursion EN2 in the uppermost member II of the Doushantuo Formation in the Yangtze Gorge area, thus favoring a biostratigraphic correlation between the Krol A assemblage and the Tanarium conoideum-Cavaspina basiconica Assemblage Zone. The Krol A data thus indicate that the “barren interval” in the Yangtze Gorges area results from a taphonomic bias due to the lack of chert nodules and may be part of the Tanarium conoideum-Cavaspina basiconica Assemblage Zone. When placed in a paleogeographic context, Ediacaran acanthomorphs from northern India, South China, and elsewhere seem to be concentrated in, if not restricted to, low paleolatitudes, with tantalizing evidence for paleobiogeographic differentiation.
The Ediacaran stratigraphic correlation between the Lesser Himalaya and the Yangtze Gorges area is definitely aided by their similarity in lithostratigraphy, taphonomy, and paleogeography, but it also demonstrates the feasibility of global correlation of Ediacaran strata using integrative data. With a refined and tested chronostratigraphic framework, it is possible to assess Ediacaran evolutionary dynamics, paleobiogeographic patterns, and environmental changes at a temporal resolution that was previously unattainable.
Acknowledgments
The research was funded by the US National Science Foundation (EAR-1124062 and EAR-2021207 to SX, EAR-1124545 to GJ, and EAR-1124303 to NCH), the National Natural Science Foundation of China (41902004 to QY and 41672027 to CZ), China Postdoctoral Science Foundation (2021M692980 to QY), and Indian University Grants Commission Basic Scientific Research Program (20-1/2012 and 20-8-12/2012 to BPS). We thank X. Shang, S. Willman, and an anonymous reviewer for constructive comments that helped improve this paper. This paper is a contribution to IGCP668.










































