Article contents
UV-resistant hydrophobic rutile titania aerogels synthesized through a nonalkoxide ambient pressure drying process
Published online by Cambridge University Press: 29 August 2012
Abstract
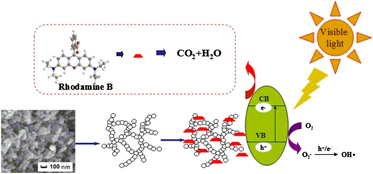
The hydrophobic rutile titania aerogels were successfully prepared by nonalkoxide ambient pressure drying through a modification process. The resulted materials were characterized by x-ray diffraction, scanning electronic microscope, transmission electron microscope, contact angle analyzer, Brunauer–Emmett–Teller specific surface area, and ultraviolet (UV)–visible diffuse reflection spectrum. The experimental results demonstrated that the as-prepared samples nanoparticles with rutile crystalline structure were uniformly distributed. The UV-resistant hydrophobic samples having high surface area were used as photocatalysts for dye degradation reaction.
- Type
- Articles
- Information
- Journal of Materials Research , Volume 28 , Issue 3: Focus Issue: Titanium Dioxide Nanomaterials , 14 February 2013 , pp. 378 - 384
- Copyright
- Copyright © Materials Research Society 2012
References
REFERENCES
- 7
- Cited by


