Published online by Cambridge University Press: 13 November 2013
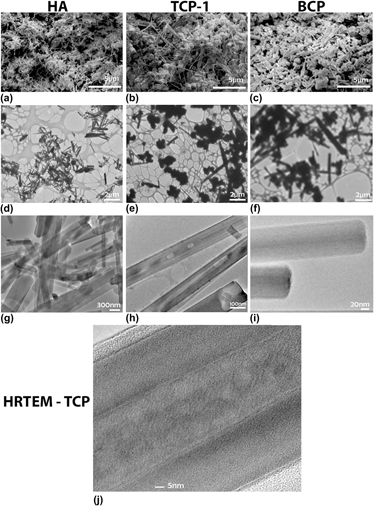
Calcium phosphates (CaPs) are major chemical constituents of mammalian bone. Their osteoconductivity in vitro and in vivo has encouraged their use in biomaterial applications such as implant materials and drug delivery. High aspect ratio nanoparticles are attractive for many biomedical applications; however, precise control of the phase and morphology is challenging. The impact of fuel-to-oxidant ratio, pH, and cation chemistry on morphology and phase was studied for CaP-based compositions by microwave-assisted solution combustion synthesis (MASCS) in a urea–nitrate (fuel–oxidant) system. An initial calcium to phosphate ratio of 1.5 was used. Highly crystalline hydroxyapatite (HA) and biphasic CaP nanoparticle compositions were produced as confirmed by x-ray diffraction, scanning electron microscopy, and transmission electron microscopy. MASCS was capable of synthesizing high aspect ratio (∼5 to 20) single and biphasic CaP nanoparticles with diameters ranging from 250 to 500 nm and lengths between 2 and 10 μm.