Article contents
Microstructure development in zinc oxide nanowires and iron oxohydroxide nanotubes by cathodic electrodeposition in nanopores
Published online by Cambridge University Press: 16 May 2011
Abstract
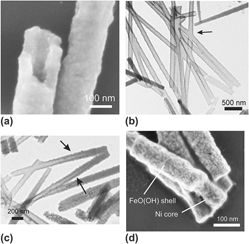
The cathodic electrodeposition of crystalline ZnO nanowires and amorphous FeO(OH) nanotubes in polycarbonate track-etched membranes with pore diameters of 50–200 nm is reported. Nitrate was used as a sacrificial precursor for the electrochemical generation of hydroxyl ions that raised the pH of the interior of the nanopore, leading to precipitation of a metal oxide or hydroxide phase. The crystalline and semiconducting ZnO phase formed directly above 60 °C at sufficiently high pH and led to the formation of dense nanowires with preferential (0001) orientation. The morphology of the wire could be influenced by the deposition temperature. Axially segmented gold–ZnO and silver–ZnO nanowires were made. In contrast, the iron hydroxide phase deposited inside the pore as a permeable gel that collapsed and transformed into hollow FeO(OH) tubes during drying. The as-formed nanotubes were amorphous and could be filled with nickel in a subsequent electrodeposition step, yielding core-shell nickel iron-oxohydroxide nanowires. The cathodic efficiency of nitrate reduction was low in both cases, suggesting that diffusional supply of metal ions may be the rate-determining step.
Keywords
- Type
- Articles
- Information
- Journal of Materials Research , Volume 26 , Issue 17: Focus Issue: Nanowires: Fundamentals and Applications , 14 September 2011 , pp. 2261 - 2267
- Copyright
- Copyright © Materials Research Society 2011
References
REFERENCES
- 11
- Cited by


