Article contents
Micrometer-sized quasicrystals in the Al85Ni5Y6Co2Fe2 metallic glass: A TEM study and a brief discussion on the formability of quasicrystals in bulk and marginal glass-forming alloys
Published online by Cambridge University Press: 16 May 2012
Abstract
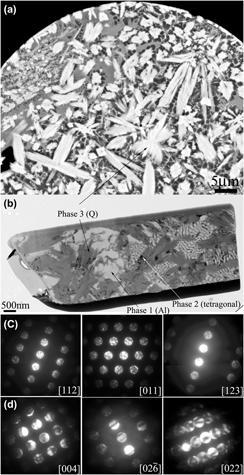
Large quasicrystals up to ∼10 μm in size with a volume fraction of ∼30% have been identified in a nitrogen gas-atomized marginal glass-forming alloy Al85Ni5Y6Co2Fe2 by detailed transmission electron microscopy. The formation of the large quasicrystal (Q) phase is discussed through the configuration of the valence electrons of its constituent elements, and the thermodynamic and kinetic factors associated with the solidification of this marginal glass-forming alloy during gas atomization. The finding leads to an important inference that marginal glass-forming alloys could be ideal systems for the formation of bulk quasicrystals under appropriate kinetic conditions. The Q phase is stable up to ∼500 °C and decomposes thereafter. The activation energy for the decomposition of the Q phase is similar to the self-diffusion of Al. Two new intermetallic phases associated with the formation and decomposition of the Q phase have also been identified and characterized.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2012
References
REFERENCES
- 9
- Cited by


