Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Du, Z.H.
Zhang, T.S.
Zhu, M.M.
and
Ma, J.
2009.
Perovskite crystallization kinetics and dielectric properties of the PMN-PT films prepared by polymer-modified sol-gel processing.
Journal of Materials Research,
Vol. 24,
Issue. 4,
p.
1576.
Ren, Zhongshan
Hu, Xiaojun
Xue, Xiangxin
and
Chou, Kuochih
2013.
Solid state reaction studies in Fe3O4–TiO2 system by diffusion couple method.
Journal of Alloys and Compounds,
Vol. 580,
Issue. ,
p.
182.
Ren, Zhong-shan
Hu, Xiao-jun
Li, Shen-yang
Xue, Xiang-xin
and
Chou, Kuo-chih
2013.
Interdiffusion in the Fe2O3-TiO2 system.
International Journal of Minerals, Metallurgy, and Materials,
Vol. 20,
Issue. 3,
p.
273.
Yin, Simin
Zhu, Yihan
Ren, Zhaohui
Chao, Chunying
Li, Xiang
Wei, Xiao
Shen, Ge
Han, Yu
and
Han, Gaorong
2014.
Facile synthesis of PbTiO3 truncated octahedra via solid-state reaction and their application in low-temperature CO oxidation by loading Pt nanoparticles.
J. Mater. Chem. A,
Vol. 2,
Issue. 24,
p.
9035.
Galizia, Pietro
and
Galassi, Carmen
2015.
Electrophoretic Deposition of Bilayer Based on Sacrificial Titanium Dioxide and Lead Zirconate Titanate on Bare Silicon Wafer.
Key Engineering Materials,
Vol. 654,
Issue. ,
p.
132.
Kiguchi, Takanori
Kodama, Yumiko
Shimizu, Takumi
Shiraishi, Takahisa
Wakiya, Naoki
and
Konno, Toyohiko J.
2019.
Interface structure of Pb(Zr,Ti)O3/MgO(001) epitaxial thin film in early stage of Stranski–Krastanov growth mode.
Japanese Journal of Applied Physics,
Vol. 58,
Issue. SL,
p.
SLLA08.
Wei, Wang
Yue, Hong-rui
and
Xue, Xiang-xin
2020.
Diffusion coefficient of Ti4+ in calcium ferrite/calcium titanate diffusion couple.
International Journal of Minerals, Metallurgy and Materials,
Vol. 27,
Issue. 9,
p.
1216.
Chen, Bojian
Jiang, Tao
Zhou, Mi
Li, Lin
Wen, Jing
and
Wen, Yongcai
2021.
Interdiffusion kinetics and solid-state reaction mechanism between Cr2O3 and calcium ferrite based on diffusion couple method.
Journal of Alloys and Compounds,
Vol. 865,
Issue. ,
p.
158754.
Quiñones-Gurrola, José Remigio
Rendón-Angeles, Juan Carlos
Matamoros-Veloza, Zully
Rodríguez-Galicia, José Luis
and
Yanagisawa, Kazumichi
2023.
Rapid one-step preparation of SrZrO3 using Zr4+ gel and SrSO4 ore under alkaline hydrothermal conditions.
Boletín de la Sociedad Española de Cerámica y Vidrio,
Vol. 62,
Issue. 5,
p.
479.
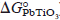 ) into the present kinetics equation, DPb2+ in the PbTiO3 interlayer was calculated with respect to the corresponding temperature. The activation energy for diffusion (Q) and the diffusion constant (D0) of Pb2+ ions in the PbTiO3 layer could be evaluated from the Arrhenius plot of DPb2+.
) into the present kinetics equation, DPb2+ in the PbTiO3 interlayer was calculated with respect to the corresponding temperature. The activation energy for diffusion (Q) and the diffusion constant (D0) of Pb2+ ions in the PbTiO3 layer could be evaluated from the Arrhenius plot of DPb2+.

