Article contents
Influence of Mn incorporation on structural, optical emission and polarization switching aspect of PbO-free nanoscale PbTiO3 systems
Published online by Cambridge University Press: 30 October 2012
Abstract
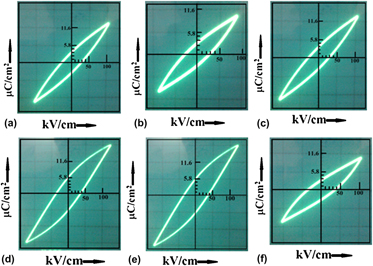
The effect of Mn incorporation into lead titanate (PbTiO3, abbreviated as PT) host lattice is being studied, and the corresponding variation with regard to tetragonality, radiative emission, and ferroelectric response is highlighted. The Mn doping has a weak dependency on the average crystallite size and is found to be within 34–39 nm for both Mn-free and Mn-included PT nanostructures. The tetragonality (c/a ratio) is found to increase with Mn level thus giving a maximum value of 1.061 for 10% Mn doping (Mn/Ti = 0.1). Further, a prominent splitting of (001) and (100) peaks in the diffractograms confirms the tetragonal characteristics of PT nanosystem. Apart from the luminescence peaks due to direct electron deexcitation and the localized states observable in the violet and blue regimes, the association of Mn2+-related orange–red emission (λ = 580 nm) was observed for Mn-incorporated PT systems. The P-E hysteresis trace exhibited a minimum tilting of ∼28° for a system that contained 10% Mn level. As a consequence of polarization switching, the effect of Mn content on remnant polarization and critical field is discussed in the light of domain wall motion and depolarization field due to surface dielectric layers. The injection of magnetic ions into nanoscale ferroelectrics is promising, which would bring insight to the understanding of charge–spin interactions for application in polarization reversal/switching elements.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2012
References
REFERENCES
- 3
- Cited by


