Article contents
Importance of line and interfacial energies during VLS growth of finely stranded silica nanowires
Published online by Cambridge University Press: 13 July 2011
Abstract
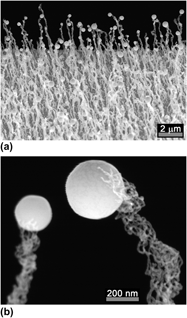
A rich research history exists for crystalline growth by vapor–liquid–solid (VLS) methods, but not for amorphous growth. Yet VLS growth in the absence of crystallographic influences provides an ideal laboratory for exploring surface energy effects, including the role of line tension. We discuss the growth of amorphous silica nanowires from indium droplets by a modified VLS method. Multiple strands issue from each droplet, each strand having <1% (i.e., < 5 nm) of the radius of the droplet. We analyze the surface forces for this system, including line tension, and combine data in a novel way to estimate the surface energy of silica, the interfacial energy of liquid indium on silica, and the line tension at the three-phase boundary. The results suggest that the growth of these silica strands would be impossible without the presence of a negative line tension that also serves to stabilize the strand radii against perturbation.
Keywords
- Type
- Articles
- Information
- Journal of Materials Research , Volume 26 , Issue 17: Focus Issue: Nanowires: Fundamentals and Applications , 14 September 2011 , pp. 2247 - 2253
- Copyright
- Copyright © Materials Research Society 2011
References
REFERENCES
- 6
- Cited by


