Article contents
Coupling effect of thermomigration and cross-interaction on evolution of intermetallic compounds in Cu/Sn/Ni ultrafine interconnects undergoing TLP bonding
Published online by Cambridge University Press: 15 May 2017
Abstract
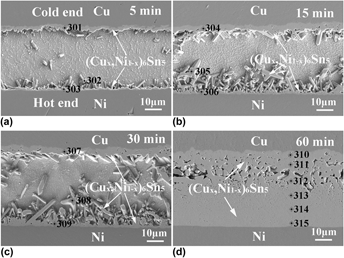
By reflowing Cu/Sn/Ni ultrafine interconnects under a temperature gradient, a new transient liquid phase (TLP) bonding process was proposed for three-dimensional packaging applications. The evolution of the dominant (Cu,Ni)6Sn5 intermetallic compounds depends strongly on the temperature gradient. The essential cause of such dependence is attributed to the different amounts of Cu and Ni atomic fluxes being introduced into the liquid solder. Under the coupling effect of thermomigration and Cu–Ni cross-interaction, the total atomic flux of Cu and Ni is promoted. As a result, the growth of dense (Cu,Ni)6Sn5 is significantly accelerated and the formation of Cu3Sn is eliminated. The new TLP bonding process consumes only a limited amount of the Ni substrate, but much more from the Cu substrate. The mechanism for the new TLP bonding process is discussed and experimentally verified in this study.
Keywords
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2017
Footnotes
Contributing Editor: C. Robert Kao
References
REFERENCES
- 8
- Cited by



