Article contents
An investigation on photoluminescence and AC powder electroluminescence of ZnS:Cu,Cl,Mn,Te phosphor
Published online by Cambridge University Press: 12 September 2011
Abstract
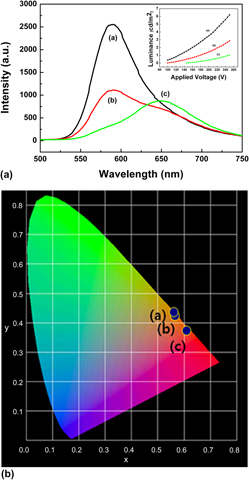
ZnS:Cu,Cl,Mn,Te, which shows red AC powder electroluminescence (ACPEL) emission, was synthesized using a conventional wet synthesis and a sealed vessel method. The photoluminescence (PL) and ACPEL were characterized. After the second firing, 0.5 wt% tellurium (Te)-doped ZnS:Cu,Cl,Mn,Te phosphor shows almost red PL emission from the 4T1–6A1 transition of Mn2+ ions, which are affected by the Te. Extended x-ray absorption fine structure analysis on the Mn K edge proved that the substitution of sulfur (S) with Te changes the local crystal field of the Mn2+ ions and shifts an orange emission (∼588 nm) to a red emission (∼650 nm). A red ACPEL emission is first shown in 0.5 wt%Te-doped ZnS:Cu,Cl,Mn,Te after the third firing phosphor even though its luminance is not very high. The origin of the ACPEL emission is assumed to be not a CuxS–ZnS p–n junction but a CuxTe–ZnS p–n junction. Raman spectra were characterized to support that the red ACPEL emission is probably attributed to a CuxTe–ZnS p–n junction.
Keywords
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2011
References
REFERENCES
- 7
- Cited by


