Article contents
A simple low-cost synthesis of brookite TiO2 nanoparticles
Published online by Cambridge University Press: 22 November 2012
Abstract
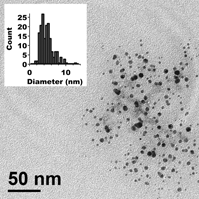
A new low-cost synthesis of brookite TiO2nanoparticles using isopropanol as both the solvent and ligand is described here. Other ligands can be bound to the titania surface during or postsynthesis to tailor the particles’ functionality. The often extremely rapid hydrolysis of titanium isopropoxide has been successfully controlled so that nanoparticle growth is achieved. The resulting 4-nm particles are nonagglomerated, stable in solution, and have a low polydispersity. The synthesis is scalable and enables the simple fabrication of large amounts of titania nanoparticles that do not scatter visible light and are highly suited for incorporation into optical composites.
Keywords
- Type
- Articles
- Information
- Journal of Materials Research , Volume 28 , Issue 3: Focus Issue: Titanium Dioxide Nanomaterials , 14 February 2013 , pp. 348 - 353
- Copyright
- Copyright © Materials Research Society 2012
References
REFERENCES
- 7
- Cited by


