Article contents
Crystal structure and oxygen nonstoichiometry of the HoxSr1−xCoO3−δ
Published online by Cambridge University Press: 30 May 2012
Abstract
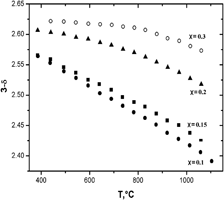
Samples with overall composition HoxSr1−xCoO3−δ within the range 0.05 ≤ x ≤ 0.9 were prepared by a solid-state technique at 1100 °C in air. Single-phase HoxSr1−xCoO3−δ oxides were obtained within the range 0.05 ≤ x ≤ 0.30. Solid solutions of Ho0.05Sr0.95CoO3−δ and Ho0.1Sr0.9CoO3−δ were indexed in the cubic structure (Pm3m sp. gr.) with the unit cell parameters a = 3.846 Å and a = 3.842 Å, respectively. Further introduction of holmium leads to a change of crystal structure from cubic to a tetragonal 2ap × 2ap × 4ap superstructure. All samples with x > 0.3 were multiphase, containing a saturated solid solution with approximate composition Ho0.3Sr0.7CoO3−δ with Ho2O3 and CoO. The change of oxygen nonstoichiometry was measured by thermogravimetric analysis within the temperature range 25 ≤ T (°C) ≤ 1100. The absolute value of oxygen nonstoichiometry was calculated from the results of chromatometric titration. Thermal expansion coefficients of Ho1−xSrxCoO3−δ were measured by dilatometry within the temperature range 25 ≤ T (°C) ≤ 1100 in air.
- Type
- Articles
- Information
- Journal of Materials Research , Volume 27 , Issue 15: Focus Issue: Advanced Materials for Fuel Cells , 14 August 2012 , pp. 2030 - 2034
- Copyright
- Copyright © Materials Research Society 2012
References
REFERENCES
- 4
- Cited by


