Introduction
Olfactory dysfunction represents one of the most frequent symptoms of coronavirus disease 2019 (Covid-19), affecting about 70 per cent of patients.Reference Spinato, Fabbris, Polesel, Cazzador, Borsetto and Hopkins1–Reference Vaira, Salzano and De Riu6 Many patients recover spontaneously within 15 days; however, severe olfactory dysfunction (i.e. anosmia and severe hyposmia) persists in 7–8 per cent of cases for over two months after clinical onset.Reference Vaira, Hopkins, Petrocelli, Lechien, Chiesa-Estomba and Salzano7–Reference Lechien, Journe, Hans, Chiesa-Estomba, Mustin and Beckers9
The pathogenesis of Covid-19 related olfactory dysfunction has not yet been elucidated.Reference Vaira, Salzano, Fois, Piombino and De Riu10 At the beginning of the pandemic, most authors hypothesised a pathogenesis linked to neuroinvasion of the olfactory bulb, with subsequent neuronal apoptosis.Reference Han, Mukdad, Long and Lopez11,Reference Chigi, Merzouki and Najimi12 This hypothesis was supported by the neuroinvasive capacity demonstrated by severe acute respiratory syndrome coronavirus-1 in the past,Reference Netland, Meyerholz, Moore, Cassell and Perlman13 and by reports of changes in the olfactory bulb on magnetic resonance imaging (MRI) in anosmic patients affected by Covid-19.Reference Politi, Salsano and Grimaldi14 However, the hypothesis was refuted by the general tendency for rapid regression of the disorder in many patients and reports that olfactory dysfunction seems to be more common in mild Covid-19 cases.Reference Yan, Faraji, Prajapati, Ostrander and DeConde15,Reference Vaira, Hopkins, Petrocelli, Lechien, Soma and Giovanditto16
For these reasons, the attention of investigators has shifted to the olfactory epithelium as a possible site of viral damage.Reference Bilinska and Butowt17 This hypothesis is further supported by: radiological evidence of olfactory cleft oedema in some anosmic patients;Reference Eliezer and Hautefort18,Reference Lechien, Michel, Radulesco, Chiesa-Estomba, Vaira and De Riu19 proof that the supporting cells of the olfactory epithelium have the highest concentration of viral receptors;Reference Lechien, Radulesco, Calvo-Henriquez, Chiesa-Estomba, Hans and Barillari20 and findings from the first two histopathological reports on animal modelsReference Bryche, St Albin, Murri, Lacote, Pulido and Ar Gouilh21 and samples taken from cadavers.Reference Kirschenbaum, Limbach, Ulrich, Rushing, Keller and Reimann22
We report the radiological and histopathological findings of a patient who presented with anosmia for more than three months after infection with severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). To the best of our knowledge, this is the first in vivo histopathology report and the first account in a patient with long-lasting anosmia.
Case report
In early March 2020, a 63-year-old woman presented with mild fever, with intense asthenia, anosmia and hypogeusia. The patient had no significant co-morbidities or previous olfactory or gustatory disturbances. In 5 days, fever and asthenia completely regressed, while chemosensory disturbances remained unchanged. At the end of March, after the detection of several cases of Covid-19 at the patient's workplace, she was subjected to a nasopharyngeal swab, which was negative, and a serological test, positive for SARS-CoV-2 immunoglobulin (Ig) IgG and IgM.
In June, given the persistence of anosmia for over three months, the patient was admitted to the maxillofacial surgery department of the University Hospital of Sassari for diagnostic investigations.
At the time of admission, nasopharyngeal swab and serological tests were repeated, which revealed positivity only for SARS-CoV-2 IgG. In the previous three months, the patient had not taken any specific medication. The olfactory and gustatory functions were objectively evaluated with psychophysical tests as per our protocol,Reference Vaira, Hopkins, Salzano, Petrocelli, Melis and Cucurullo2,Reference Vaira, Deiana, Fois, Pirina, Madeddu and De Vito23,Reference Vaira, Salzano, Petrocelli, Deiana, Salzano and De Riu24 which detected anosmia and severe hypogeusia.
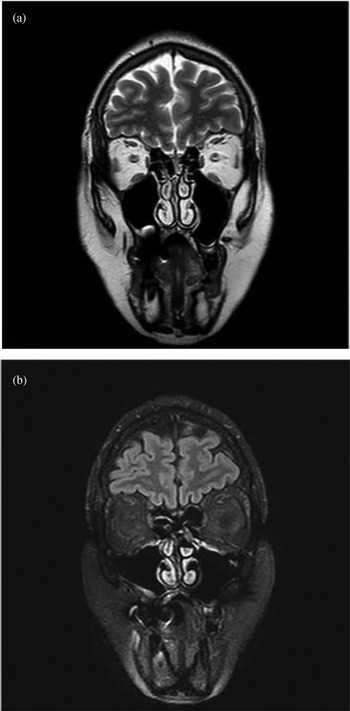
The patient was first subjected to contrast-enhanced MRI of the nasal cavities and brain. The examination did not reveal any pathological findings: the olfactory bulb and clefts were of normal volume, without signal anomalies (Figure 1).

Fig. 1. Magnetic resonance imaging did not reveal any pathological findings: the olfactory bulb and clefts were of normal volume, without signal anomalies. Coronal scans of: (a) T2-weighted fast spin echo sequence, and (b) T2-weighted fluid-attended inversion recovery with fat suppression sequence.
After providing signed written consent and being informed about the risks of the procedure, the patient underwent a biopsy of the left olfactory epithelium. The endoscopic procedure was conducted under general anaesthesia, as previously described by other authors.Reference Andrews, Poirrier, Lund and Choi25 During the procedure, a swab was performed directly on the olfactory epithelium, which showed as negative for residual Covid-19.
Histopathological findings
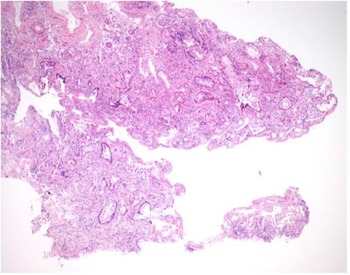
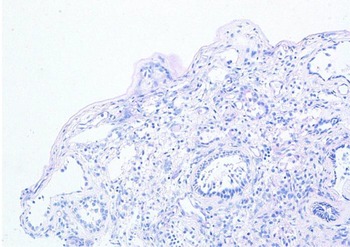
The mucosal biopsy sections measured 8 mm × 4 mm in maximum dimension. There was extensive loss of surface epithelium (Figure 2), with no associated surface fibrin or inflammatory exudate (Figure 3). The architecture of glands in the lamina propria was maintained. A minimal chronic lymphocytic inflammatory infiltrate was present (Figure 2). No eosinophils or mast cells were identified.

Fig. 2. Low power stain shows mucosa devoid of surface epithelium. There is mild chronic inflammation, but no evidence of acute inflammation. (H&E; ×25)

Fig. 3. Special stain does not highlight surface basement membrane or inflammatory exudate. (Periodic acid–Schiff; ×100)
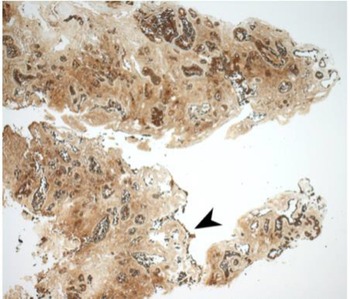
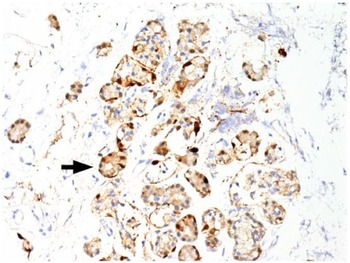
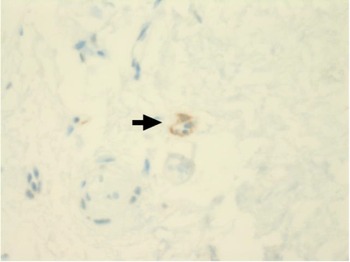
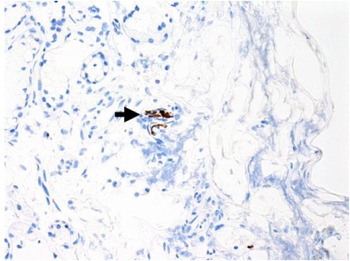
Immunohistochemical staining for pan-cytokeratin AE1/AE3 antibodies demonstrated only very focal residual attenuated surface epithelium (Figure 4). There was strong nuclear and cytoplasmic positivity for S100 immunostain in scattered cells within structures, compatible with Bowman's glands (Figure 5); the same immunostain highlighted small nerve bundles, possibly of trigeminal origin. Immunostaining for angiotensin-converting enzyme 2 (ACE2) receptor showed focal membrane staining in the S100 positive cells in Bowman's glands (Figure 6). There was focal positive staining for synaptophysin, and neurofilament immunostain highlighted small neurites and nerve bundles in lamina propria (Figure 7). No abnormal neural proliferation was identified.

Fig. 4. Immunostain showed possible attenuated residual surface epithelial cells, stained brown (arrowhead). (Pan-cytokeratin immunostain; ×25)

Fig. 5. Immunostain shows strong nuclear and cytoplasmic positivity in scattered cells in structures compatible with Bowman's glands (arrow). The same immunostain highlighted small nerve bundles, possibly of trigeminal origin, not illustrated in this field. (S100 immunostain; ×200)

Fig. 6. Immunostaining for angiotensin-converting enzyme 2 (ACE2) receptor showed focal membrane staining in cells that were also positive for S100 in Bowman's glands (arrow). (ACE2 immunostain; ×200)

Fig. 7. Focal positive staining for neurofilament immunostain highlighted small neurites and nerve bundles in lamina propria (arrow). (Neurofilament immunostain; ×100)
Based on the results of the histopathological examination, the patient began systemic cortisone therapy with prednisone, starting with 75 mg/day and tapering the dose for 15 days. The patient received nasal irrigation with betamethasone, ambroxol, and Rinazina® for 30 days. At the end of therapy, the patient reported a slight improvement in chemosensory symptoms. Psychophysical tests revealed severe hyposmia and moderate hypogeusia. A new cycle of cortisone therapy was scheduled for September.
Discussion
Nasal congestion associated with viral infections of the upper respiratory tract often causes transient anosmia.Reference Seiden26 However, the olfactory dysfunction in Covid-19 is not characteristically associated with rhinitis symptoms; the cause is therefore more likely to be due to injury to the olfactory epithelium or olfactory apparatus than secondary to nasal obstruction. The exact location of this damage remains uncertain given the paucity in the literature of histopathological studies on samples taken from Covid-19 patients.
In the case reported here, in which anosmia persisted for three months after Covid-19 infection, the MRI findings excluded any macroscopic inflammation affecting the olfactory bulb, the pathway or the olfactory epithelium (Figure 1). These findings are consistent with those of Galougahi et al.,Reference Galougahi, Ghorbani, Bakhshayeshkaram, Naeini and Haseli27 but are in contrast with reports from other authors, who detected olfactory cleft inflammationReference Eliezer and Hautefort18,Reference Lechien, Michel, Radulesco, Chiesa-Estomba, Vaira and De Riu19 or an increase in olfactory bulb volumeReference Politi, Salsano and Grimaldi14 in the early stages of anosmia. Our radiological findings suggest that an aetiology linked to olfactory bulb impairment is unlikely. Only a bulbar biopsy, clearly impossible in patients recovered from Covid-19, could rule out a macroscopically non-evident nerve injury.
Kirschenbaum et al.Reference Kirschenbaum, Limbach, Ulrich, Rushing, Keller and Reimann22 reported on the post-mortem histological analysis of olfactory epithelium in two elderly male patients who died 6 and 8 days after hospital admission. They demonstrated findings consistent with an inflammatory neuropathy, with prominent leukocytic infiltrates in the lamina propria, focal atrophy of the mucosa, and digestion chambers in the olfactory nerve fibres suggestive of axonal damage. Both brains showed perivascular leukocytic infiltrates, predominantly in the basal ganglia and intravascular microthrombi.
Using a mouse model to study the effects of SARS-CoV-2, Bryche et al.Reference Bryche, St Albin, Murri, Lacote, Pulido and Ar Gouilh21 demonstrated extensive olfactory epithelium damage within days of inoculation, almost exposing the olfactory sensory neurones. The virus was shown to be present in the olfactory epithelium at day 2, but was already decreasing by day 4. The virus was not demonstrated in the olfactory bulb or cortex. In keeping with the work of Brann et al.,Reference Brann, Tsukahara, Weinreb, Lipovsek, Van den Berge and Gong28 Bryche et al.Reference Bryche, St Albin, Murri, Lacote, Pulido and Ar Gouilh21 demonstrated infection of the supporting sustentacular cells, but not the olfactory neurons themselves. Desquamation, however, affected both infected and non-infected cells, with the olfactory neurons showing loss of cilia.
The ACE2 receptor is considered the portal of entry for SARS-CoV-2, and upregulation of ACE2 receptors may increase the risk of infection.Reference Hoffmann, Kleine-Weber, Schroeder, Kruger, Herrler and Erichsen29 No upregulation was detected in our patient's biopsy.
Previous studies in patients with non-coronavirus post-viral olfactory loss show long-lasting changes in the olfactory epithelium. Yamagishi et al.Reference Yamagishi, Hasegawa and Nakano30 showed thinning of the epithelium with loss of the characteristic three-layer structure; there was also a reduction in the number of olfactory receptor cells, while those that were present lacked cilia. Patients with hyposmia demonstrated more ciliated olfactory neurons. In anosmic patients, olfactory vesicles were absent; in hyposmic patients, they were reduced in number.Reference Moran, Jafek, Eller and Carter Rowley31 Jafek et al.Reference Jafek, Murrow, Michaels, Restrepo and Linschoten32 demonstrated patchy regeneration of the olfactory epithelium interspersed with respiratory epithelium, and in some cases the olfactory epithelium was replaced by metaplastic squamous epithelium.
These reports are all consistent with our results. The findings suggest that disruption and desquamation of the olfactory epithelium is the underlying mechanism in Covid-19 related olfactory dysfunction. Failure of epithelial repair leads to thinning and loss of the olfactory dendrites. Patchy recovery may lead to hyposmia and/or dysosmia.
• A patient presented with anosmia for more than three months after severe acute respiratory syndrome coronavirus-2 infection
• The patient was first subjected to contrast-enhanced magnetic resonance imaging of the nasal cavities and brain
• The examination did not reveal any pathological findings: the olfactory bulb and clefts were of normal volume, without signal anomalies
• A biopsy, taken three months after onset of coronavirus disease 2019 related anosmia, demonstrated massive olfactory epithelium disruption
• These findings shift the focus away from olfactory bulb invasion and towards treatments targeted at surface epithelium
The findings have important implications when considering novel treatment options that could be targeted to the olfactory epithelium. There is evidence to support steroid rinses, but not intranasal steroid sprays;Reference Yan, Overdevest and Patel33 this may reflect the greater ability of steroid rinses to reach the olfactory epithelium. In a small pilot study,Reference Yan, Mundy and Patel34 submucosal injection of platelet rich plasma into the olfactory epithelium in seven patients with hyposmia was associated with significant improvement, but no benefit was found in two anosmic patients. There was no control arm in that study, but further study is certainly warranted. A number of other topical agents have been investigated in small studies, but in a recent evidence-based review none were considered to provide sufficient evidence about which to make any treatment recommendations.Reference Hura, Xie, Coby, Schlosser, Orlov and Seal35 Given the large numbers of patients affected, this must be made a research priority.
Conclusion
The biopsy, taken three months after the onset of Covid-19 anosmia, demonstrated massive disruption of the olfactory epithelium. This shifts the focus away from olfactory bulb invasion and encourages further studies of treatments targeted at the surface epithelium.
Acknowledgements
The authors wish to thank Prof Alessandro Franchi for his valuable comments on the interpretation of the histopathological analysis. When we informed the patient that the biopsy could reveal irreparable damage to the olfactory epithelium, she replied that if this could help others to prevent or treat this long-term morbidity then it would be worth it. This pandemic has allowed us to be enlightened by many gestures of small and great altruism, like this one. To all these ‘Covid-19 heroes’ goes our deepest gratitude and admiration.
Competing interests
None declared