Article contents
Pressure distribution over the leaflets and effect of bending stiffness on fluid–structure interaction of the aortic valve
Published online by Cambridge University Press: 28 November 2019
Abstract
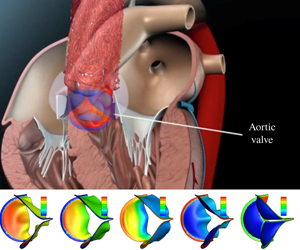
We describe a three-dimensional (3-D) fluid–structure interaction (FSI) simulation study of the aortic valve, where the flow is driven by a specified transient pressure drop along the aorta tube. The thickness of the leaflets is varied from 0.05 mm to 0.8 mm so that the normalized bending rigidity by the systolic pressure gradient covers a wide range from  $1.5\times 10^{-4}$ to 0.6. The non-uniform pressure distribution over the leaflets and the transient valve force are calculated, including the ‘water hammer’ effect during rapid closure. With low bending rigidity, the valve functions normally and produces physiological characteristics of healthy valves. However, exceedingly low rigidity leads to flapping motion of the leaflets and, in some cases (e.g. the normalized bending rigidity around 0.001), may reduce the performance index. As the leaflets become much stiffer, the valve is more difficult to open and slower to close, which leads to higher resistance and a reduced flow rate. Therefore, our results suggest that there is an optimal range of bending rigidity for the valve, roughly between 0.003 and 0.04 in normalized term. We further develop a one-dimensional unsteady flow model based on the momentum and mass conservation equations to replace the 3-D flow in the FSI simulation. The new flow model incorporates pressure loss across the valve as well as the leaflet motion. Comparison with the 3-D results shows that the reduced flow model is able to produce a reasonable 3-D deformation sequence of the leaflets, opening area and flow rate, especially in the cases of low bending rigidity.
$1.5\times 10^{-4}$ to 0.6. The non-uniform pressure distribution over the leaflets and the transient valve force are calculated, including the ‘water hammer’ effect during rapid closure. With low bending rigidity, the valve functions normally and produces physiological characteristics of healthy valves. However, exceedingly low rigidity leads to flapping motion of the leaflets and, in some cases (e.g. the normalized bending rigidity around 0.001), may reduce the performance index. As the leaflets become much stiffer, the valve is more difficult to open and slower to close, which leads to higher resistance and a reduced flow rate. Therefore, our results suggest that there is an optimal range of bending rigidity for the valve, roughly between 0.003 and 0.04 in normalized term. We further develop a one-dimensional unsteady flow model based on the momentum and mass conservation equations to replace the 3-D flow in the FSI simulation. The new flow model incorporates pressure loss across the valve as well as the leaflet motion. Comparison with the 3-D results shows that the reduced flow model is able to produce a reasonable 3-D deformation sequence of the leaflets, opening area and flow rate, especially in the cases of low bending rigidity.
JFM classification
- Type
- JFM Papers
- Information
- Copyright
- © 2019 Cambridge University Press
References
Chen et al. supplementary movie 1
Chen et al. supplementary movie 2
Transient valve deformation and corresponding force on the valve for h=0.3 mm.
Chen et al. supplementary movie 3
Valve deformation and corresponding vortex structures in the flow during systole for h=0.3 mm
- 10
- Cited by




