As healthcare decision makers continue to face challenges in health services delivery to their patient population with increasing costs and limited resources, disinvestment programs and initiatives are being established internationally as one approach to maintain a sustainable healthcare system (Reference Chambers, Salem, D'Cruz, Subedi, Kamal-Bahl and Neumann1–Reference Orso, de Waure and Abraha4). Numerous disinvestment programs were established after 2006 (Reference Orso, de Waure and Abraha4). The results of these disinvestment programs and activities are mixed. Suggestions to improve their success include stakeholder engagement throughout the disinvestment process and the promotion of its potential benefits to the clinical community as a way to provide quality patient care more efficiently (Reference Chambers, Salem, D'Cruz, Subedi, Kamal-Bahl and Neumann1;Reference Lim, Heng, Tai, Tham and Chua2).
In 2015, the Swiss Federal Office of Public Health (FOPH) established a health technology assessment (HTA) program, which focuses on the re-evaluation of benefits that are covered by the compulsory health insurance. HTAs facilitate transparent, evidence-based decision making, help to reduce ineffective and inefficient services, medicines and processes as well as raise the quality of healthcare. By using a defined process, potential low-value services or technologies (e.g., medical devices, services and procedures, drug therapies, and laboratory tests) that are deemed to lack efficacy, appropriateness or cost-effectiveness are identified.
Since the establishment of this program, numerous potentially obsolete services and technologies have been nominated for reassessment by HTA every year. Until 2017, an informal exchange with other HTA agencies with a similar interest helped to inform the identification of potential re-evaluation topics during the pilot phase of this program. As there is a growing interest to establish an information exchange and collaboration on the identification of potential disinvestment candidates and projects among organizations that are involved or are interested in learning about ongoing disinvestment initiatives, this study was proposed by the FOPH to the Health Technology Assessment International Disinvestment & Early Awareness Interest Group (HTAi DEA IG).
This study aimed to collect data and information by means of a survey of disinvestment candidates and ongoing disinvestment projects from members of the HTAi DEA IG, members of the EuroScan International Network (EuroScan), and members of the International Network of Agencies for Health Technology Assessment (INAHTA). The HTAi DEA IG aims to be a key international center for sharing knowledge and expertise (knowledge-hub), both in methods for prioritizing and assessing obsolete or low-added value technologies and in the practical application of disinvestment for health systems (5).
EuroScan is a scientific association and network of public agencies, scientific organizations and individuals for sharing and collecting information and development of methods for the early identification, appropriate use, and awareness of health technologies in their life cycle. As well, INAHTA's mission is to provide a forum for the identification and pursuit of interests that are important to its member agencies (6).
The main study objectives were twofold: (i) to identify disinvestment candidates through information sharing with international organizations, mainly HTA units, and (ii) to share the knowledge, experiences, and challenges with ongoing disinvestment projects. This online survey was run as a pilot with the aim to foster the international collaboration and information exchange amongst countries and institutions with an interest in disinvestment activities.
For our survey, disinvestment refers to the processes of (partially or completely) withdrawing health resources from any existing healthcare practices, procedures, technologies or pharmaceuticals that are deemed to deliver little or no health gain for their cost and, thus, are not efficient health resource allocations (Reference Elshaug, Hiller, Tunis and Moss7). Disinvestment is distinct from reassessment, which is the evidence-based assessment of the clinical, social, ethical, and economic impact of a health technology in clinical practice to inform its use compared with standard care (Reference Soril, Niven, Esmail, Noseworthy and Clement8).
Methods
Survey Sample
In May 2018, members of the HTAi DEA IG and members of EuroScan and INAHTA received an invitation by means of email to participate in the survey. The survey was conducted from May 18 until June 14, 2018. A reminder email was sent 2 weeks before the deadline.
Invited members were informed that the privacy of their responses would be protected and that any information provided would be used in a peer-reviewed journal.
Survey Questionnaire
An online survey was developed using “Encuesta Facil”© (https://www.encuestafacil.com/) software to collect information from the HTAi DEA IG, INAHTA, and EuroScan members on their organization's disinvestment activities and candidates. A maximum of ten technologies could be described in the questionnaire.
A draft survey was pilot-tested among the HTAi DEA IG Executive Team, and the final version was distributed to all HTAi DEA IG, EuroScan, and INAHTA members (Supplementary File 1).
Data Analysis
Responses were collated in an electronic database. Two investigators independently reviewed all responses for clarity, completeness, and analysis (I.G.I. and J.P.). For responses that were unclear or incomplete, one investigator followed-up with the survey respondent. Frequencies of responses were calculated for close-ended questions, and responses to open-ended questions were summarized narratively. The unit of analysis was the HTAi DEA IG member organization.
Results
Survey Response Rate
We invited 362 individuals to participate in the survey, and we received responses from 27 respondents (response rate: 7.5 percent). If two individuals from the same organization completed the survey, we followed up with them to verify the responses and ensure that there were no contradictions. The responses were then consolidated to represent one organization. Our sample size, therefore, was based on unique responses representing twenty-four organizations. Almost 70 percent of these were involved in disinvestment initiatives.
Descriptive Statistics
Survey Respondent Characteristics
Table 1 outlines the demographics of the respondents who completed the survey. In the case of several responses per organization, a consolidated response was considered.
Table 1. Survey Respondent Characteristics (N = 24)

a Respondents from these countries indicated that they were not involved in disinvestment activities.
Three respondents each represented Australia and the United Kingdom, two each represented Canada, Germany, Norway, Russia, and Spain, and one each were from Switzerland, Brazil, India, Italy, Netherlands, Romania, Finland, and Turkey. The majority of respondents represented the public sector (83.3 percent; 20/24). Three were from the private sector, and one was independent.
Almost seventy percent of respondents participated in disinvestment activities (66.7 percent; 16/24). Although one HTA agency indicated that they were not involved in disinvestment activities, they have produced reports on disinvestment topics and have a disinvestment working group within their organization.
Activities in Disinvestment Process
Among the respondents engaged in disinvestment activities (N = 16), 75 percent (12/16) were involved in assessing the health technologies, 62.5 percent (10/16) partook in the topic identification, in prioritization and in decision making. Other activities included topic selection (56.3 percent; 9/16), dissemination (50 percent; 8/16), coordination and implementation (43.8 percent; 7/16) (Table 2). Respondents were able to select more than one disinvestment activity in which their organization is involved.
Table 2. Activities Addressed in Disinvestment Process by Survey Respondenta Involved in Disinvestment Activities (N = 16)
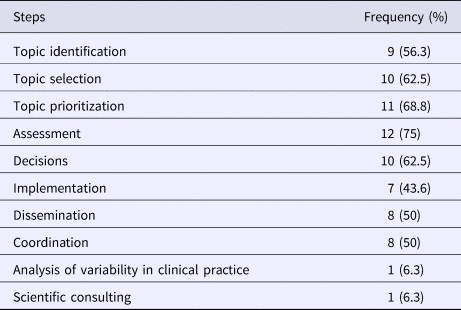
a Respondents were able to select more than one response.
Disinvestment Candidates
Twenty-seven disinvestment candidates (n = 26 unique disinvestment candidates) were identified in the survey responses (Supplementary Table 1). They can be organized into four categories: surgical or medical procedures (e.g., knee arthroscopy for osteoarthritis) (n = 15), drugs (e.g., diacerein) (n = 5), medical devices (e.g., mechanical chest compression devices) (n = 4), and laboratory tests (e.g., vitamin D tests) (n = 2). One respondent from the private sector did not identify the health technologies proposed for disinvestment due to confidentiality issues. Furthermore, tonsillectomy was listed twice by the same respondent, but the indications for the intervention were different in each instance. Another respondent specified that the disinvestment candidates were derived from Choosing Wisely Australia.
The targeted populations and indications were closely linked with the use of the health technology in question. The patient population encompassed pediatrics, pregnant women, women, and men affected by specific indications and the general public. For instance, there are no conditions for vitamin D tests in Switzerland, and decision makers may impose limitations.
The frequency of use for the proposed disinvestment candidates per year ranged from approximately fifty surgeries to remove pacemaker leads in adults in Australia (year not indicated) to 970,000 vitamin B12 tests performed in 2015 in Switzerland.
The spread for the estimated annual costs associated with the use of disinvestment candidates was between EUR 400,000 for diacerein in patients with osteoarthritis, and EUR 75–105 million for proton pump inhibitors for patients with nonerosive gastroesophageal reflux disease (Supplementary Table 2). As the estimated cost savings are dependent primarily on the disinvestment decision, they were not available for disinvestment candidates without any decision yet.
Reasons for Proposed Disinvestment
The most common reason for the proposed disinvestment candidate is due to evidence or signaling of clinical ineffectiveness (n = 21), followed by evidence or suspicion of inappropriate use (n = 20), and availability of an alternative health technology with better clinical effectiveness (n = 10). Other reasons include evidence or signaling of inadequate safety (n = 9), cost reduction (n = 6), and availability of an alternative with better cost-effectiveness (n = 3). Low compliance with the medical device use, variability of the medical intervention in some jurisdictions, and low sensitivity and specificity related to the diagnostic curettage prompted decision makers to propose some health technologies as disinvestment candidates (Supplementary Table 1). Respondents were able to select more than one reason for each proposed disinvestment candidate.
Disinvestment Decisions
Among the twenty-seven disinvestment candidates, stakeholders limited their use in clinical practice for eleven of them (40.7 percent; 11/27) and completely removed five health technologies (18.5 percent; 5/27). Decisions for eleven of the disinvestment candidates (40.7 percent; 11/27) had not been determined during the survey completion (Supplementary Table 1).
Methods, Frameworks, or Tools Used for Disinvestment Decisions on a Health Technology
The most common approach used for disinvestment decisions of a health technology was health technology assessments (43.8 percent; 7/16), health technology reassessments (37.5 percent; 6/16), and the Guideline for Not Funding Health Technologies (GuNFT) (12.5 percent; 2/16). Respondents also indicated that they used multi-criteria decision analysis, the Model for Sustainability in Health Care by Allocating Resources Effectively, evidence reviews, clinician engagement, and an atlas of variability in medical practice (Table 3) (Reference Bryan, Mitton and Donaldson9).
Table 3. Methods, Frameworks, or Tools Used for Disinvestment Decision on a Health Technology since 2015 (N = 16)
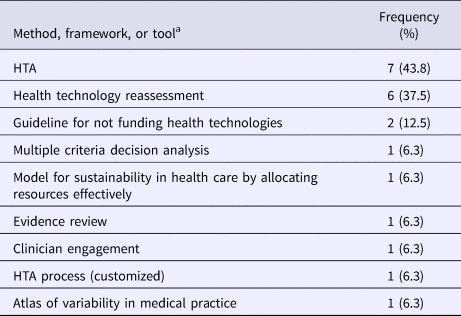
HTA, health technology assessment.
a Respondents were able to select more than one response.
Barriers to Disinvestment Decisions
Based on the respondents’ answers, barriers experienced by their organization in reaching a decision on the disinvestment of a health technology include the strength of well-established interest and advocacy groups (43.8 percent; 7/16), lack of relevant data to conduct an assessment (37.5 percent; 6/16), lack of a systematic decision process for disinvestment (31.2 percent; 5/16), and political challenges (31.2 percent; 5/16) (Table 4). Other barriers selected were sunk costs with the existing technology (31.3 percent; 5/16), uncertainty about the perceived benefits with disinvestment (18.8 percent; 3/16), conflicting priorities among stakeholders (18.8 percent; 3/16), difficulty in resource reallocation (18.8; 3/16), and sensitivities surrounding the disinvestment target population (12.5 percent; 2/16). Respondents were able to select more than one barrier.
Table 4. Barriers to Disinvestment Decisions and their Implementation (N = 16)

a Respondents were able to select more than one response.
Barriers to the Implementation of Disinvestment Decisions
Noted barriers to implementing disinvestment decisions were the clinician's reluctance to remove practices that they perceive as integral to their professional practice and identity (31.3 percent; 5/16), lack of funding for implementation (25 percent; 4/16), lack of incentives 18.8 percent; 3/16), insufficient timelines to implement decisions (12.5 percent; 2/16), loss of perceived benefit related to the removal of the technology (12.5 percent; 2/16), perception that disinvestment is a cost saving exercise only (12.5 percent; 2/16), and lack of skills in change management (n = 6.3 percent; 1/16). Three respondents were not involved in the implementation of disinvestment decisions, and the disinvestment program is new for one respondent, so no disinvestment decisions have been implemented yet (Table 4). Respondents were able to select more than one barrier.
Content Analysis on Open-Ended Question
Respondents had the opportunity to submit their comments on disinvestment candidates and activities. Among the twenty-four survey respondents, eight responded to the open-ended question.
One respondent mentioned that clinical practice guidelines and recommendations in “do not do lists” are also important sources for identifying disinvestment candidates. Another respondent commented that many decisions on disinvestment candidates result in limited use versus complete removal. This observation aligns with our survey findings, where 65.4 percent (17/26) of decisions led to limited use of the health technology in question.
In Russia, a program dedicated to disinvestment does not exist. Instead, the inclusion of interventions, including drug therapies, onto the Essential Drug List is based on cost-effective analyses with alternative therapies. The creation of an effective system to invest in healthcare is perceived as challenging.
Although CADTH indicated that it does not have a formal disinvestment program, the agency has published reports that have addressed disinvestment and are publicly available. As well, a working group was assembled to explore approaches to position the organization to contribute to advancing disinvestment initiatives for other health technologies. The agency, however, continues to seek opportunities to collaborate with other organizations and researchers that are active or interested in disinvestment activities.
Discussion
Our survey collected information on disinvestment candidates and activities from members of the HTAi DEA IG, the EuroScan International Network, and INAHTA. Of the 362 invited members, we received twenty-four unique responses. Among our survey respondents, approximately 70 percent were involved in disinvestment initiatives.
The disinvestment candidates identified in the survey represented an array of health technologies. The majority of the suggested candidates were medical and surgical procedures (n = 15), followed by drugs (n = 5), medical devices (n = 4), and laboratory tests (n = 2). The reason for this unequal distribution of candidates was not captured in the survey, but could be based on the existence of well-established evaluation processes for certain technologies in various countries. In most countries, drug therapies receive approval for market access after undergoing a formal evaluation of efficacy and safety. In contrast, the market approval process for medical devices, services, and procedures are different.
In the Swiss healthcare system, for instance, medical services provided by physicians or chiropractors are automatically covered by the mandatory health insurance (according to the “principle of trust”) unless a stakeholder requests an assessment of the service based on a reasonable suspicion of a lack of effectiveness, efficacy, appropriateness, and cost-effectiveness (Reference De Pietro, Camenzind and Sturny10). Evidence or signaling of clinical ineffectiveness or inappropriate use typically resulted in the nomination of health technologies for disinvestment.
Health technology assessments (Reference Bryan, Mitton and Donaldson9) and reassessments (Reference Soril, Niven, Esmail, Noseworthy and Clement8) were the most frequent methods used to evaluate the technology for proposed disinvestment, and, in many cases, the decisions led to the limited use of the disinvestment candidate. While the strength of interest and advocacy groups, lack of relevant data to conduct an assessment, lack of a systematic decision process and political challenges were some of the barriers to disinvestment decisions, clinician reluctance, and lack of funding and incentives were obstacles to the implementation of disinvestment decisions. Similar barriers to disinvestment decisions and implementation listed by the survey respondents were also noted by Harris et al. (Reference Harris, Green and Elshaug11). Participants in a 2012 survey by Leggett et al. on health technology reassessment programs indicated that political challenges and lack of expertise in the field were barriers that they had encountered. To mitigate these barriers, stakeholder engagement was an effective approach (Reference Leggett, MacKean, Noseworthy, Sutherland and Clement12).
The survey findings suggested that additional factors, such as the volume and data availability and stakeholder perspectives, play a significant role in the disinvestment decision process. In Switzerland, a health technology qualifies for a re-evaluation in their HTA program if there is an initial suspicion of a lack of efficacy, effectiveness, appropriateness, or cost-effectiveness. These concerns must be supported by an initial evidence review. Stakeholders are then involved in the topic identification and prioritization process and are consulted for the HTA report.
In the topic prioritization phase, the available data about disinvestment candidates are limited due to time and resource constraints for information retrieval. Prioritizing nominated disinvestment candidates before an HTA provides the underlying information, is perceived by many stakeholders as a dilemma. Nevertheless, prioritization criteria play an integral part in the ranking of HTA topics, and a criterion like potential cost savings plays amongst others a significant role in this process. As a disinvestment decision on the technology has not been made yet, potential cost savings can be difficult to estimate. In Spain, disinvestment candidates are typically identified not only due to a lack of perceived efficacy, safety, and comparative cost-effectiveness, but also when variability in practice exists (13). Decisions are then made on the basis of established procedures in some regions, such as the GuNFT guideline (Reference Ibargoyen-Roteta, Gutiérrez-Ibarluzea and Asua14). Irrespective of the growing interest in disinvestment and reassessment, consensus on a disinvestment framework at an international level remains outstanding (Reference Calabró, La Torre and de Waure15).
Limitations
Our study is not without limitations. Given the number and geographical dispersion of the HTAi DEA IG, EuroScan, and INAHTA members, an online survey was conducted to collect data and information on their organization's disinvestment activities and candidates. Although some members of the HTAi DEA IG, INAHTA, and EuroScan have an interest in disinvestment, the response rate was less than 10 percent (7.5 percent), so it is very likely that the response rate may be reflective of the member organization's activity level in disinvestment, confidentiality and privacy concerns with sharing their information, or lack of time to provide details on their disinvestment activities and candidates. As well, the survey asked explicit questions about the processes and frameworks used to reach a disinvestment decision. It is possible that the member organizations make disinvestment decisions informally or use a more passive approach (i.e., reduce the use of an older technology over time in favor of a newer technology), but this information was not captured in the survey responses. Explicit knowledge on existing initiatives and results from exploring the webpages of EuroScan and INAHTA members, however, do suggest that no additional formal programs are in place.
Directions for Future Research
A qualitative study using interviews with survey respondents, who are engaged in disinvestment activities, may be warranted to learn more about the processes, frameworks, and methods used and facilitators for disinvestment prioritization, and disinvestment decision making and implementation. To ease the feasibility of information exchange on disinvestment in the HTA community, an exploration of the interest in, value of, and efforts to expand existing HTA registries to include assessment reports on disinvestment candidates and decisions may be warranted. The study can also explore the interest and feasibility in establishing and sustaining an international forum for organizations that are faced with disinvestment decisions.
In addition, members of the HTAi DEA IG will develop a literature search strategy to identify any recent published literature on disinvestment and reassessment, including case studies, new processes or frameworks developed, and discussion papers. The literature search will be conducted on a quarterly basis, and the relevant citations will then be presented in a newsletter and posted on the HTAi DEA IG webpage.
As well, our survey revealed that an important barrier with the implementation of a disinvestment decision centers on a clinician's unwillingness to remove a health technology or medical procedure that they deem necessary for their clinical practice. Future research, therefore, can investigate the feasibility of developing partnerships between clinicians involved with Choosing Wisely and the HTA community to support disinvestment initiatives. The Choosing Wisely campaign was established in 2012. Medical specialty societies involved in the campaign ask their members to identify tests or procedures that are overused in routine practice. Lists are then created and updated according to explicit guidelines outlined by Choosing Wisely, and they are intended to help support conversations between clinicians and patients to help avoid unnecessary care (16). In Spain, the Dissemination of Initiatives to Analyse Appropriateness in Healthcare (DIANAHealth) initiative is intended to improve the appropriateness of care and clinical practice through the identification of potentially obsolete or low-value interventions and developing recommendations to reduce their use. It also seeks to promote the use of better options if available. DIANAHealth is a subproject of Clinical Epidemiology Program of the Centro de Investigación Biomédica en Red de Epidemiología y Salud Pública (CIBERESP). CIBERESP is a research network in Spain that is comprised of health professionals and researchers from academia and hospitals with a focus to develop public health policies (17). A qualitative study that observes the relationships between healthcare professionals and researchers in this network, reviews the methods and processes to develop recommendations, and interviews healthcare professionals on their experiences and perspectives on implementing these recommendations also warrants further investigation.
Conclusions
To conclude, the survey results suggest that disinvestment activities are occurring in a limited manner in the HTA community. Disinvestment candidates include drug therapies, medical devices, laboratory tests, and medical and surgical procedures. Health technologies or procedures are commonly nominated for disinvestment due to evidence or signaling of clinical ineffectiveness or inappropriate use. In most instances, the decisions led to the limited use of the disinvestment candidate according to our survey responses. Given the survey response rate (7.5 percent), it is highly likely that among the HTAi DEA IG, EuroScan, and INAHTA members more disinvestment activities are ongoing and more candidates are nominated or processed. Future research can explore the disinvestment prioritization process, frameworks and methods used among our survey respondents in a qualitative study, and explore opportunities and approaches to form closer ties between the HTA and clinical communities involved with Choosing Wisely or DIANAHealth with the intent to increase the implementation of disinvestment decisions.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0266462319000229
Author ORCIDs
Iñaki Gutierrez-Ibarluzea, 0000-0002-4080-7963.
Conflicts of interest
The authors do not declare any conflicts of interest.








