To the Editor—We wish to point out that 12 months into the coronavirus disease 2019 (COVID-19) pandemic, with >111 million reported cases and >2.4 million deaths, many knowledge gaps still need to be resolved empirically to fully appreciate the risks associated with high-touch environmental surface (HITES) contamination. It has been argued that indirect transmission through contaminated HITES is an unlikely route of transmission for severe acute respiratory syndrome coronavirus virus 2 (SARS-CoV-2) (eg, Goldman Reference Goldman1 and Meyerowitz et al Reference Meyerowitz, Richterman and Gandhi2 ) Why? Is this position based on data, and if so what data?
The World Health Organization 3 rightly has made the point that it is difficult to separate potential direct and indirect exposure in establishing transmission relevancy. The safest approach is, therefore, to avoid discounting the possibility of indirect transmission until proper studies have been performed to support this view. Reference Cai, Sun and Huang4,Reference Kanamori5 Specifically, if we are to rule out indirect transmission as a likely route, we should do so based on adequate supporting data. What data do we need? The scenario in Figure 1 illustrates the primary knowledge gaps.
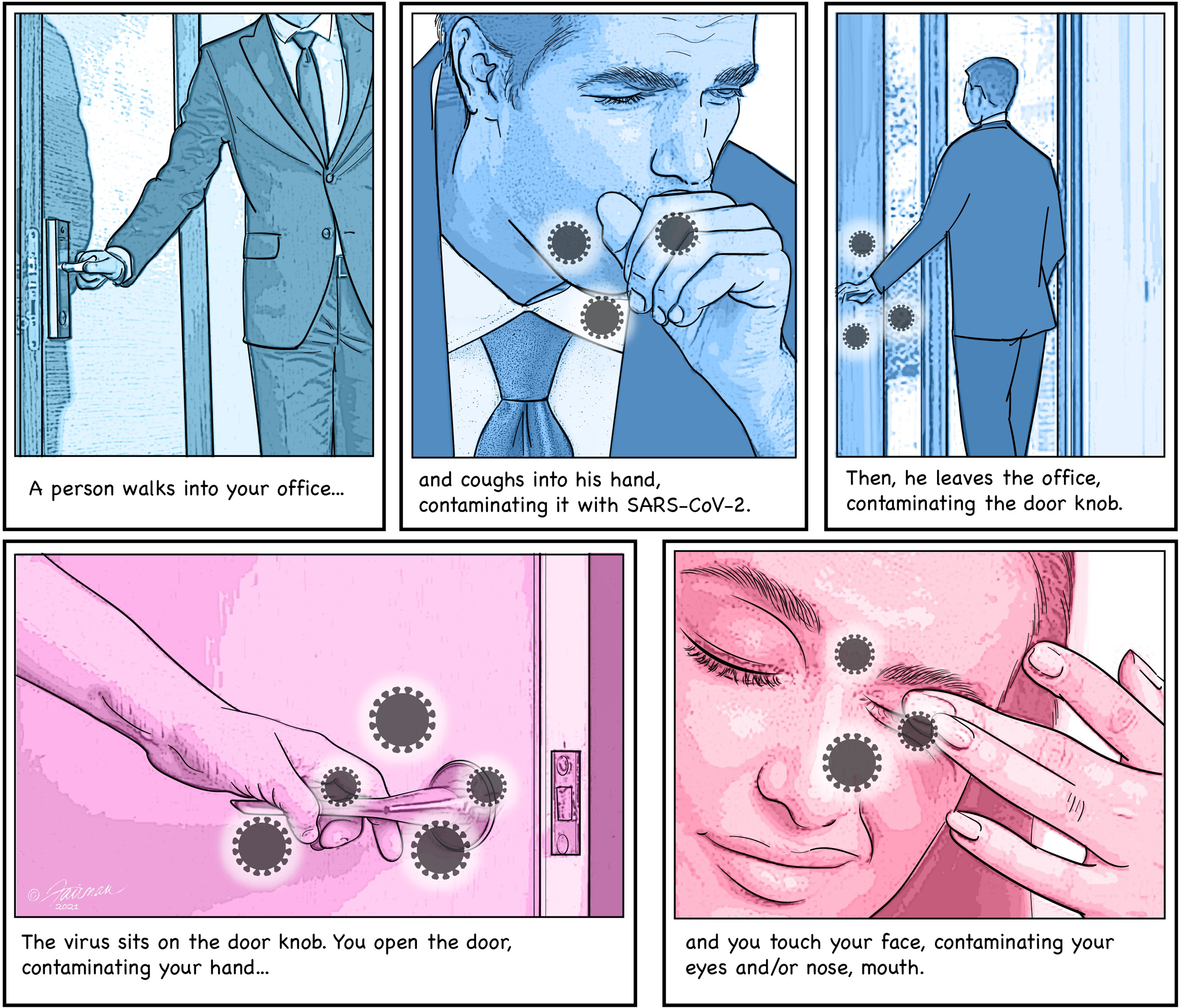
Fig. 1. Example scenario for an indirect transmission of SARS-CoV-2 from one person to another through virus-contaminated HITES.
What does our current knowledge tell us about the risk of acquiring infectious SARS-CoV-2 during the scenario illustrated in Figure 1? Unfortunately, the extent of deposition of infectious SARS-CoV-2 (not viral RNA determined by PCR assay!) onto the visitor’s hand when he coughs, has not been empirically determined. On the basis of the accepted respiratory droplets or aerosol transmission route, one must assume that a significant burden of infectious virus would be discharged onto the hand by such a cough. If so, why haven’t the data been generated to support this? We do know that SARS-CoV-2 can survive on skin for hours (half-life of 3–5 hours at room temperature). Reference Hirose, Ikegaya and Naito6,Reference Harbourt, Haddow and Piper7 Assuming that the SARS-CoV-2–infected visitor leaves the office within the hour, the virus deposited on his hand while coughing should remain infectious until he reaches for the door knob. Here we run into another knowledge gap, for we have no empirical data to help us assess the quantity of infectious SARS-CoV-2 that might be transferred from the visitor’s hand to the door knob. Once on the steel door knob, we do, however, have empirical data to help us predict how long infectious SARS-CoV-2 can remain there. For instance, half-life data on SARS-CoV-2 survival on experimentally contaminated prototypic HITES at room temperature exist from several investigators. Reaching for the contaminated door knob as you leave the office that day, there will likely be infectious SARS-CoV-2 remaining, per the half-life data for steel (19 to 143 hours at room temperature, depending on the organic matrix in which the SARS-CoV-2 was deposited). Reference Hirose, Ikegaya and Naito6,Reference Ijaz, Nims and Zhou8 How much infectious SARS-CoV-2 will be transferred to your hand as you reach for the door knob? We do not have empirical data to allow us to assess this. Nor do we know the quantity of SARS-CoV-2 that might be transferred to your susceptible mucous membranes (ocular, nasal, oral) as you self-inoculate on the way out of the office. Finally, we simply do not yet know how much SARS-CoV-2 must be introduced to a susceptible host’s mucous membranes to initiate infection (ie, the human minimal infectious dose).
For the moment, let us take the position that transmission via respiratory droplets and/or aerosols is the only relevant mode of SARS-CoV-2 transmission. Are we on a stronger footing from a data perspective? Do we, for instance, know how much infectious SARS-CoV-2 is discharged as we speak, breath, sneeze, or cough? Unfortunately, no related data are yet available. Some data on the survival of SARS-CoV-2 in aerosols have been reported, Reference van Doremalen, Morris and Holbrook9 but the totality and quality of these data are not nearly as complete as are the surface survival data, reflecting the challenges in performing such experiments. We can use the half-life value of 1.2 hours Reference van Doremalen, Morris and Holbrook9 for our purposes. There is still debate over what constitutes a safe distance for avoiding possible SARS-CoV-2 transmission. It is likely >2 m, Reference Setti, Passarini and De Gennaro10 but are we sure? Finally, we are back to the question of how much infectious SARS-CoV-2 must be introduced into a mucous membrane via portals of entry to initiate an infection. Many knowledge gaps, therefore, apply also to the direct transmission pathway.
It is generally believed that no strong empirical data support an indirect route of infectious SARS-CoV-2 dissemination. Reference Goldman1,Reference Meyerowitz, Richterman and Gandhi2 We would like to turn this argument on its head; namely, do we have enough empirical data to rule out an indirect transmission route for infectious SARS-CoV-2? In the interest of public safety, we strongly encourage investigators to begin closing these knowledge gaps.
Acknowledgments
We acknowledge Jennifer Fairman for creating the figure. We also thank Dr Chris Jones and Dr Mark Ripley, both from Reckitt Benckiser R&D, for their critical review of the manuscript and feedback.
Financial support
Reckitt Benckiser funded the preparation of the manuscript and the figure.
Conflicts of interest
Raymond Nims received a fee from Reckitt Benckiser for writing and editing the manuscript. The other authors report no conflicts of interest relevant to this article.






