Introduction
BPIFA2 (BPI fold-containing family A, member 2) (previously named Parotid Secretory Protein – PSP, SPLUNC2, C20orf70) is an abundant protein in mouse saliva (Nandula et al., Reference Nandula, Huxford, Wheeler, Aparicio and Gorrin press) and Bpifa2 is highly expressed in the three paired major salivary glands (Gao et al., Reference Gao, Oei, Ovitt, Sincan and Melvin2018). BPIFA2 is structurally related to LPS-binding proteins and the salivary protein binds LPS and contains an LPS-blocking peptide (Abdolhosseini et al., Reference Abdolhosseini, Sotsky, Shelar, Joyce and Gorr2012). Indeed, we recently characterized a Bpifa2 knockout mouse and found that BPIFA2 is the major surfactant protein in mouse saliva and BPIFA2 depletion affects the activity of ingested LPS, insulin secretion and the metabolomics profile (Nandula et al., Reference Nandula, Huxford, Wheeler, Aparicio and Gorrin press). Our recent report left a number of alternate functions for BPIFA2 unexplored. In this report we show that BPIFA2 may also play a role in sodium intake and blood pressure regulation.
Objective
The physiological function of BPIFA2 remains uncertain but the protein is down-regulated 6-fold in male spontaneously hypertensive rats compared to normotensive WKY rats (Zhang et al., Reference Zhang, Zhong, Wang, Liu, Cong, Xiang, Wu, Yu and Zhang2017). The objective of this study was to determine if BPIFA2 expression affects blood pressure in a Bpifa2 knockout mouse model. Since sodium intake is associated with hypertension, the study also determined the effect of BPIFA2 depletion on sodium preference in this mouse model.
Methods
Mouse colony
Animal experiments were reviewed and approved by the University of Minnesota Institutional Animal Care and Use Committee. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional guides on the care and use of laboratory animals. Bpifa2tm1(KOMP)Vlcg (RRID: MMRRC_046819-UCD) were obtained from the KnockOut Mouse Project at UC Davis (https://www.komp.org/) and bred and housed as described (Nandula et al., Reference Nandula, Huxford, Wheeler, Aparicio and Gorrin press). Different groups of mice were used for the blood pressure and sodium preference experiments.
Blood pressure and heart rate monitoring
Mice were acclimatized in the CODA system (Kent scientific, Torringtron, CT) for 3 days prior to experiments. Blood pressure was monitored in 6 mice per group with a tail cuff. Each mouse was tested for up to 23 cycles and data cycles accepted as defined by the instrument. Heart rate was monitored simultaneously with blood pressure.
Sodium intake
Mice (18 males per group; 8 females per group) were acclimatized to two water bottles with free access to food. The mice were then provided one bottle with pure water and one bottle that contained 0.15 M NaCl. Consumption from both bottles was calculated after 3 days at which time their position was switched and consumption again calculated after 3 days. % salt preference was calculated as (ml salt water consumed / total water consumption) x 100%.
Results
BPIFA2 differentially alters blood pressure in male and female mice
To test the effect of BPIFA2 on BP, this was monitored in groups of mice and compared between male and female, WT and KO mice. Systolic (Fig. 1A) and diastolic (Fig. 1B) BP did not differ between WT male and female mice. However, both readings were significantly lower in male KO mice compared to male WT mice. In contrast, BP was increased in female KO compared to both female WT mice and male KO mice (Fig. 1). The heart rate did not differ between experimental groups (Fig. 2).
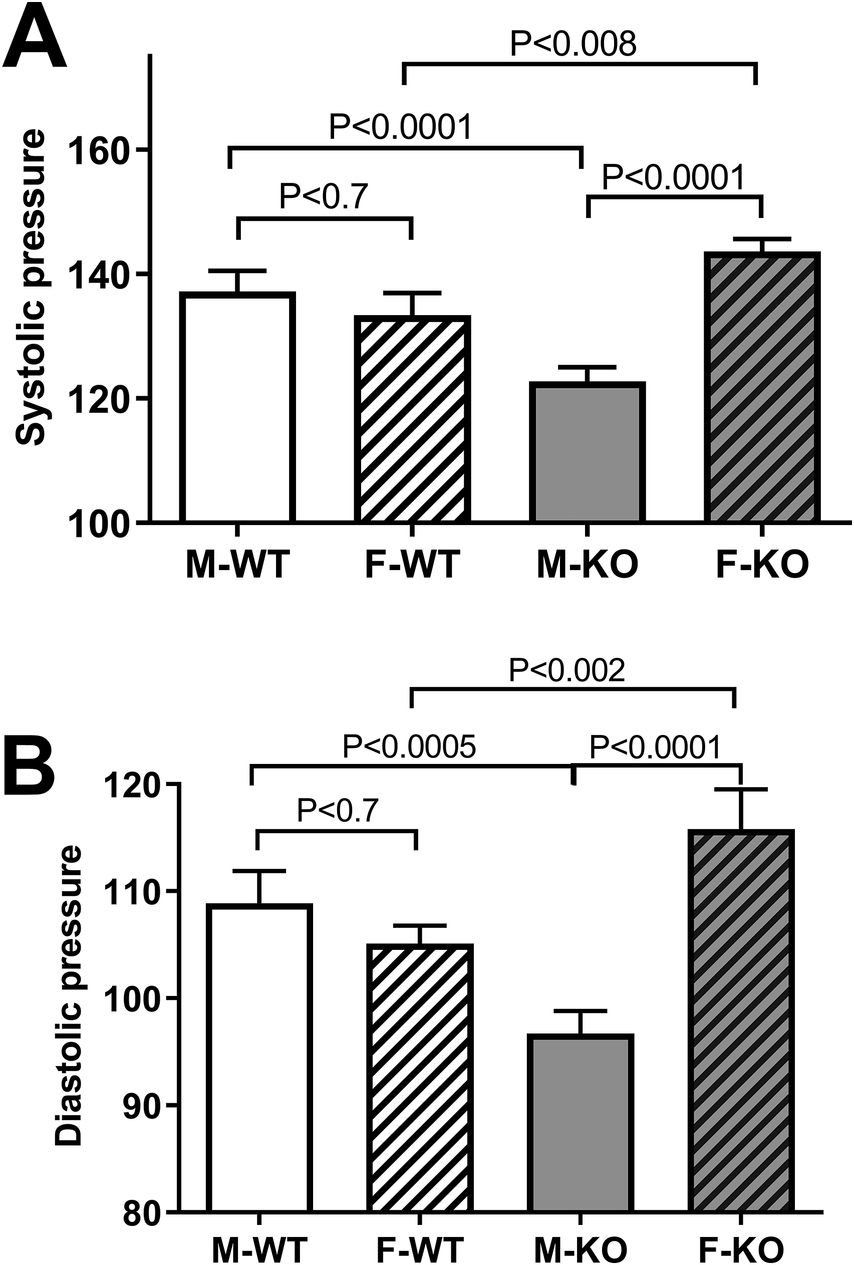
Figure 1. Blood pressure. A. systolic BP in Male (M) and Female (F), WT and KO mice. Six mice per group, 13–18 readings/mouse, N = 79–107. B. diastolic BP in the same groups as in panel A. Data are shown as mean ± 95% confidence intervals. The groups were compared by one-way ANOVA with Sidaks post-test for multiple comparisons.

Figure 2. Heart rates. Heart rates (BPM) were recorded during the blood pressure determination in the same groups as in Fig. 1. Each point represents one mouse. Data were analyzed by one-way ANOVA with Tukey’s multiple comparisons post-test. P > 0.4 for all pairwise comparisons.
BPIFA2 differentially alters salt preference in male and female mice
Female WT mice showed a significant preference for 0.9% saline compared to male WT mice. This difference was reduced in the absence of BPIFA2, suggesting that this protein affects sodium preference (Fig. 3).
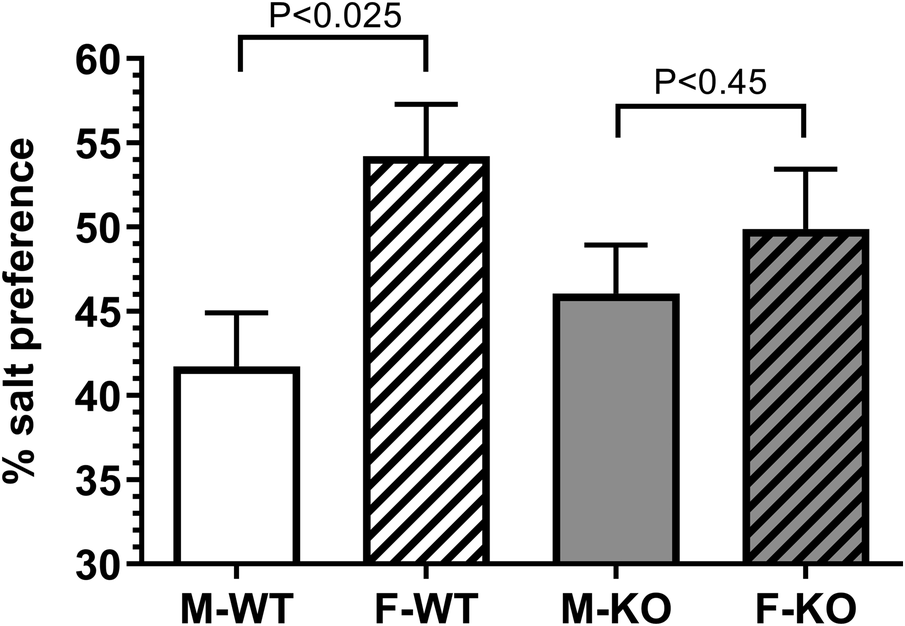
Figure 3. Sodium preference. The preference for 0.15 M NaCl was determined in duplicate by two-bottle cross-over test against pure water. N = 18 (M), N = 8 (F). Data are shown as mean ± 95% CI and were analyzed by Student’s t-test for each genotype. P-values are shown.
Discussion
Depletion of BPIFA2 leads to decreased blood pressure in male mice. Thus, the decreased BPIFA2 expression reported in male hypertensive rats (Zhang et al., Reference Zhang, Zhong, Wang, Liu, Cong, Xiang, Wu, Yu and Zhang2017) may be a compensatory response to the higher blood pressure in these rats. The reverse effect of BPIFA2 depletion in female mice suggests that the effect of BPIFA2 is sex dependent.
Conclusions
We previously reported that BPIFA2 binds LPS (Abdolhosseini et al., Reference Abdolhosseini, Sotsky, Shelar, Joyce and Gorr2012) and affects the activity of ingested LPS and metabolic endotoxemia (Nandula et al., Reference Nandula, Huxford, Wheeler, Aparicio and Gorrin press). In addition, renal stem/progenitor cells respond to an LPS insult by secretion of BPIFA2 (Sallustio et al., Reference Sallustio, Stasi, Curci, Divella, Picerno, Franzin, Palma, Rutigliano, Lucarelli, Battaglia, Staffieri, Crovace, Pertosa, Castellano, Gallone and Gesualdo2019). The results presented here expand the systemic effects to sodium intake and blood pressure control. It remains to be determined if these effects are mediated by BPIFA2 binding of oral LPS. The oral microbiome is a source of oral LPS, which could affect blood pressure and sodium intake. In this context, the recent finding that changes in the oral microbiome are linked to hypertension in post-menopausal women (Gordon et al., Reference Gordon, LaMonte, Genco, Zhao, Li, Hovey, Tsompana, Buck, Andrews, Mcskimming, Zheng, Sun and Wactawski-Wende2019) suggests an effect of sex hormones on oral LPS levels, which could mediate the observed effects on blood pressure. LPS also regulates epithelial sodium channels (Baines et al., Reference Baines, Albert, Hazell, Gambling, Woollhead and Dockrell2010), which have been implicated in sodium preference (Roper, Reference Roper2015) and are regulated by BPIFA2 (Tarran et al., Reference Tarran, Stutts and Donaldson2012). The results presented here point to a novel pathway for blood pressure regulation that originates in the oral cavity.
Acknowledgements
I thank Dr. Pilar Ariza Guzmán, University of Minnesota Integrative Biology and Physiology Phenotyping Cores and the staff of the University of Minnesota Research Animal Resources for assistance with the blood pressure experiments and mouse colony husbandry, respectively.
Author Contributions
SUG conceived and designed the study, conducted data gathering, performed statistical analyses and wrote the article.
Funding Information
This work was supported by research funds from the University of Minnesota School of Dentistry, which is gratefully acknowledged. No other specific grant from any funding agency, commercial or not-for-profit sectors were received for this research.
Conflicts of Interest
The author declares no conflicts of interest.
Data Availability Statements
Raw data files are available from the author upon request, subject to institutional approval.








Comments
Comments to the Author: This short paper brings brief results on the blood pressure, heart rates and sodium preference in BPIFA2 knock-out mice compared to wild-type mice. The most interesting result is the sex-dependent effect of BPIFA2 depletion on blood pressure, i.e. males knock-out mice decrease their blood pressure whilst females knock-out increase. Authors showed that the salt preference is also sex dependent in wild-type mice whilst in knock-out mice the sex difference is no longer significant. Their claim that reduced blood pressure correlates with increased sodium preference and oppositely, increased blood pressure decrease sodium preference should be further tested because there is no statistical evidence supporting this claim. If this minor problem is explained or corrected in the MS, I find the paper acceptable as it provides new views on potential roles of this interesting protein in the regulation of the blood pressure.