Introduction
Suicide is a leading cause of death all over the world, accounting for 1.3% of all global deaths in 2019 (World Health Organisation, 2021a). In the USA, suicide was the 10th leading cause of death in 2019, and the second leading cause in young people (National Institute of Mental Health, 2022), although 77% of suicides occur in low- or middle-income countries (World Health Organisation, 2021b). Suicide attempts are at least 20 times as common as suicide (World Health Organisation, 2022), and are especially frequent among young people (O'Connor et al., Reference O'Connor, Wetherall, Cleare, Eschle, Drummond, Ferguson, O'Connor and O'Carroll2018; Sivertsen et al., Reference Sivertsen, Hysing, Knapstad, Harvey, Reneflot, Lonning and O'Connor2019). They cause substantial service use and economic costs (Royal College of Psychiatrists, 2010; Hawton et al., Reference Hawton, Casanas, Haw and Saunders2013) and are also a strong predictor of subsequent suicide (Chan et al., Reference Chan, Bhatti, Meader, Stockton, Evans, O'Connor, Kapur and Kendall2016).
Mood disorders increase the risk of suicide and suicide attempts. Long-term data suggest around 3% of people hospitalised with a diagnosis of bipolar disorder subsequently die by suicide, and 1.5% of those diagnosed with unipolar depression (Nordentoft et al., Reference Nordentoft, Mortensen and Pedersen2011). Suicide attempts and self-harm are also more common in people with mood disorders (Weintraub et al., Reference Weintraub, Van de Loo, Gitlin and Miklowitz2017).
Lithium was first suggested to have anti-suicidal properties by Mogens Schou in 1954 (Schou et al., Reference Schou, Juel-Nielsen, Stromgren and Voldby1954), and the claim was repeated in the 1970s in a well-known report on the links between suicide and mental disorder (Barraclough, Reference Barraclough1972). More recently, several leading researchers have supported the idea that lithium can prevent suicide, based initially on cohort studies (Baldessarini et al., Reference Baldessarini, Tondo, Davis, Pompili, Goodwin and Hennen2006). An influential meta-analysis of randomised trials also concluded that lithium can reduce the risk of suicide (Cipriani et al., Reference Cipriani, Hawton, Stockton and Geddes2013). Moreover, ecological analyses have reported associations between lithium levels in drinking water and lower suicide rates (Memon et al., Reference Memon, Rogers, Fitzsimmons, Carter, Strawbridge, Hidalgo-Mazzei and Young2020), although publication bias and heterogeneity are noted as limitations in the latest meta-analysis of these studies (Eyre-Watt et al., Reference Eyre-Watt, Mahendran, Suetani, Firth, Kisely and Siskind2021). Thus, despite some recent analyses coming to different conclusions (Riblet et al., Reference Riblet, Shiner, Young-Xu and Watts2017; Borjesson and Gotzsche, Reference Borjesson and Gotzsche2019), many researchers and clinicians regard lithium's anti-suicidal properties as ‘proven’ (Lewitzka et al., Reference Lewitzka, Jabs, Fulle, Holthoff, Juckel, Uhl, Kittel-Schneider, Reif, Reif-Leonhard, Gruber, Djawid, Goodday, Haussmann, Pfennig, Ritter, Conell, Severus and Bauer2015a, p. 1) by ‘unambiguous evidence’ (Lewitzka et al., Reference Lewitzka, Severus, Bauer, Ritter, Muller-Oerlinghausen and Bauer2015b, p. 1). Lithium has been suggested to have an ‘intrinsic anti-suicidal property’ (Del Matto et al., Reference Del Matto, Muscas, Murru, Verdolini, Anmella, Fico, Corponi, Carvalho, Samalin, Carpiniello, Fagiolini, Vieta and Pacchiarotti2020), and is recommended in some clinical practice guidelines for the prevention of suicidal behaviour (Veterans Affairs and Department of Defence, (2019). There have been calls to make guideline recommendations more assertive (Smith and Cipriani, Reference Smith and Cipriani2017) and for lithium to be added to drinking water (Daly, Reference Daly2020).
Drawing conclusions about a rare event such as suicide is always difficult, however, and previous studies suffer from methodological limitations. Most importantly, all meta-analyses conducted to date used the Peto method, which excludes studies in which no outcome events occur, and has recently been suggested to be problematic for this reason (Ren et al., Reference Ren, Lin, Lian, Zou and Chu2019). Since suicide is so rare, many trials with relevant data have not been included in these analyses, which may have inflated treatment effects. We set out to conduct a meta-analysis of the effects of lithium on suicide and non-fatal suicidal behaviour using data from all eligible trials.
Methods
This systematic review was conducted following guidance from the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for best-practice standards (Page et al., Reference Page, McKenzie, Bossuyt, Boutron, Hoffmann, Mulrow, Shamseer, Tetzlaff, Akl, Brennan, Chou, Glanville, Grimshaw, Hrobjartsson, Lalu, Li, Loder, Mayo-Wilson, McDonald, McGuinness, Stewart, Thomas, Tricco, Welch, Whiting and Moher2021). The protocol was developed and registered prior to data extraction (PROSPERO ID: CRD42021265809; https://www.crd.york.ac.uk/prospero/display_record.php?RecordID = 265809).
Search strategy
PubMed, PsycINFO, Embase, ClinicalTrial.gov and the Cochrane Schizophrenia Group trial register were searched using the following terms: [‘lithium’] AND [‘affective disorder*’ OR ‘mood disorder*’ OR ‘depress*’ OR ‘bipolar’ OR ‘schizoaffective’ OR ‘personality disorder’ OR ‘dysthymia’ OR ‘rapid cycling’] AND [‘randomised control trial’ OR ‘RCT’ OR ‘trial’ OR ‘random*’]. In addition to terms for bipolar disorder and depression, additional mood diagnostic categories were included to ensure no relevant papers were missed (see online Supplementary Table S1). Searches were conducted up until March 2022. Google Scholar was searched to identify previous reviews and meta-analyses of similar topics, and a hand-search of the reference lists allowed for further studies to be identified. For protocols or ongoing trials, the researchers were contacted to determine if the studies had been completed and were near publication.
Study selection
Inclusion criteria consisted of randomised control trials published in peer-reviewed literature that compared lithium with placebo or treatment as usual in the medium and long-term (>12 weeks) treatment of mood disorders. We included studies that employed treatment as usual, in which participants were not blinded, since we suspected that even when a placebo is used, blinding is likely to be inadequate due to the noticeable side effects of lithium. Participants were required to be over the age of 18 years and to have a diagnosis of a mood disorder made clinically or according to standardised diagnostic criteria manuals. Studies needed to be published after the year 2000 due to more modern reporting requirements introduced by the CONSORT statement (Begg et al., Reference Begg, Cho, Eastwood, Horton, Moher, Olkin, Pitkin, Rennie, Schulz, Simel and Stroup1996) ensuring completeness of reporting. In older trials, suicide may not always have been reported, and since suicide is so rare, even one or two unreported events would have a substantial impact on the analysis. No language restrictions were applied.
Trials conducted to evaluate relapse prevention in mood disorders and trials specifically aimed at prevention of suicide and suicidal behaviour were eligible for inclusion. Trials in which lithium was used alone or in conjunction with other treatments were included where other treatments were available to the control group too. Comparative studies comparing lithium with another active drug that did not include a placebo or treatment as usual group were excluded.
Study selection
All titles and abstracts identified by the search were screened by ZN and independently checked by JM. Full-text screening of the remaining studies was conducted by ZN and a sample was double checked by JM. Any uncertainties relating to inclusion were discussed and resolved with JM and JS.
Quality assessment
The risk of bias in the included studies was assessed using an operationalised version of the original Cochrane Risk of Bias tool (Higgins and Green, Reference Higgins and Green2011) for consistency with a previous influential meta-analysis (Cipriani et al., Reference Cipriani, Hawton, Stockton and Geddes2013). This measure rates studies as being at ‘high’, ‘low’ or ‘unclear’ risk of bias according to various domains. Criteria for the rating of each domain were established by consensus within the research team in accordance with the Cochrane guidelines (online Supplementary Table S2). Quality assessments were carried out independently by ZN, JM and JS and then compared and discrepancies were resolved through discussion and consensus.
Data extraction
Data were extracted from eligible studies by ZN and JM; uncertainties were resolved by discussion including the third author (JS). The following data were extracted; year of publication; region; duration; interventions; participants' age and inclusion diagnosis; other treatments; previous treatment with lithium; aim of the study and data on suicide and suicidal behaviour, including suicide attempts. In cases of missing data or areas of uncertainty, the corresponding author of the paper was contacted. For trials in which suicide was not reported and a response was not received, it was assumed that no suicides occurred.
Outcomes and analysis
The primary outcome of interest was suicide. Secondary outcomes were non-fatal suicidal behaviour, as defined by each individual study (this could include suicidal ideation – see Table 1). Due to the heterogeneity of definitions of non-fatal outcomes used in the individual studies, we also decided to look at suicide attempts specifically.
Table 1. Characteristics of studies included in this systematic review

a Figures for suicides confirmed by authors.
b Suicidal behaviour included: suicide attempts, interrupted suicide attempts and hospitalisation to prevent suicide.
There is no consensus about the methods for meta-analysis of rare events (Efthimiou, Reference Efthimiou2018). Although the Peto method is the standard procedure in meta-analyses of categorical data (Cochrane Collaboration, 2022), this method may not perform well when the number of trials with zero events is increasing, for unbalanced designs, when the log odds ratio (OR) significantly differs from zero, or when there is no true effect size (Cheng et al., Reference Cheng, Pullenayegum, Marshall, Iorio and Thabane2016; Ren et al., Reference Ren, Lin, Lian, Zou and Chu2019). The Peto method may overestimate the treatment effect when there are more trials with zero events in the treatment condition compared to the control condition (Dahabreh and Economopoulos, Reference Dahabreh and Economopoulos2008). Since suicide is rare, and does not occur at all in many trials, the Peto method excludes the majority of available data on lithium and suicide, and may lead to overestimation of the size of a treatment effect (Sharma et al., Reference Sharma, Gotzsche and Kuss2017).
Various other methods have been proposed to facilitate analysis of rare and zero events, but most involve approximations, such as continuity corrections, which are not recommended (Efthimiou, Reference Efthimiou2018) and produce parameters that are difficult to interpret (Lane, Reference Lane2013). For this reason, it is common to simply pool the data, for example in regulatory assessments of adverse events (Lievre et al., Reference Lievre, Cucherat and Leizorovicz2002; Bradburn et al., Reference Bradburn, Deeks, Berlin and Russell Localio2007). We therefore conducted an analysis of the pooled data using the two-sided Fisher's Exact test in R for the comparison of proportions. Following publication of the protocol, we also decided to conduct meta-analysis of ORs, using the Exact method and Bayesian methods, which have been recommended as methods for including trials with zero events (Ren et al., Reference Ren, Lin, Lian, Zou and Chu2019). We prioritised the Exact method by Liu et al. (Reference Liu, Liu and Xie2014) over the Bayesian method because the latter may be sensitive to the choice of prior distributions. All analyses were done in R 4.2.0 (R Core Team, 2022). We used the gmeta-package for the Exact method and the rstan/MetaStan packages for the Bayesian analyses. We applied a sceptical, non-informative prior (1/250 < OR < 250) and an informative prior (1/15 < OR < 15). Ninety-five per cent confidence intervals (or credible intervals in the Bayesian analyses) were calculated for each pooled OR, and individual study results and meta-analysis results were displayed in Forest plots. Additionally, we used other meta-analytical methods, including the Peto method, as sensitivity analyses. The R-code is available via the Open Science Framework (https://osf.io/6q3w7/).
For suicide we used data from all trials and assumed that if suicide was not reported then no suicides had occurred. This assumption is reasonable as all trials were published since 2000, after publication of reporting guidance such as the CONSORT statement that specifies the reporting of important harms (Begg et al., Reference Begg, Cho, Eastwood, Horton, Moher, Olkin, Pitkin, Rennie, Schulz, Simel and Stroup1996). As a sensitivity analysis, we looked at trials which explicitly reported suicides or in which we had been able to confirm the occurrence of suicides with the authors. For comparison with previous meta-analyses, we also conducted a sensitivity analysis including trials published before 2000, using data extracted in a previous meta-analysis (Cipriani et al., Reference Cipriani, Pretty, Hawton and Geddes2005).
Heterogeneity of effect sizes was estimated, if possible, with I 2 and τ and the respective 95% confidence intervals. Subgroup analyses were also performed, following Cochrane guidelines to explore heterogeneity between subgroups when estimating treatment effects based on sparse event data (Cochrane Collaboration, 2022). We performed subgroup analyses involving trials in which lithium was used specifically to prevent suicide; trials that did not include people who were taking lithium prior to randomisation; trials involving people with bipolar disorder; and trials involving people with depression spectrum disorder or mixed diagnostic groups.
We applied the same analysis to the secondary outcomes of non-fatal suicidal behaviour and suicide attempts, but we only included trials which explicitly reported on suicidal behaviour in this analysis, since, unlike suicide, we cannot assume this behaviour would be routinely reported.
Results
Sample and trial characteristics
A total of 447 citations were identified after removal of duplicates. After screening, 12 trials published between 2000 and 2021 were identified that satisfied inclusion criteria (see Fig. 1 and online Supplementary Table S3 for details of excluded studies). Table 1 outlines included trial characteristics. Three trials were designed to examine the effect of lithium on suicide and non-fatal suicidal behaviour specifically, and nine trials explored lithium's effect on relapse in people with bipolar disorders or depression. Nine trials reported on suicide explicitly and seven on other suicidal behaviour. The most common comparator was placebo, used in ten trials, and the other two compared lithium with usual care or, in one case, a personalised treatment approach available to all participants (‘optimalised personalised treatment’) (Nierenberg et al., Reference Nierenberg, Friedman, Bowden, Sylvia, Thase, Ketter, Ostacher, Leon, Reilly-Harrington, Iosifescu, Pencina, Severe and Calabrese2013). Six trials included participants with bipolar disorder exclusively and six trials included participants with a diagnosis of depression or mixed ‘affective spectrum’ disorders. Follow-up times ranged from 20 to 104 weeks. Six trials included only two arms, while the remaining six trials also compared lithium to another active drug.
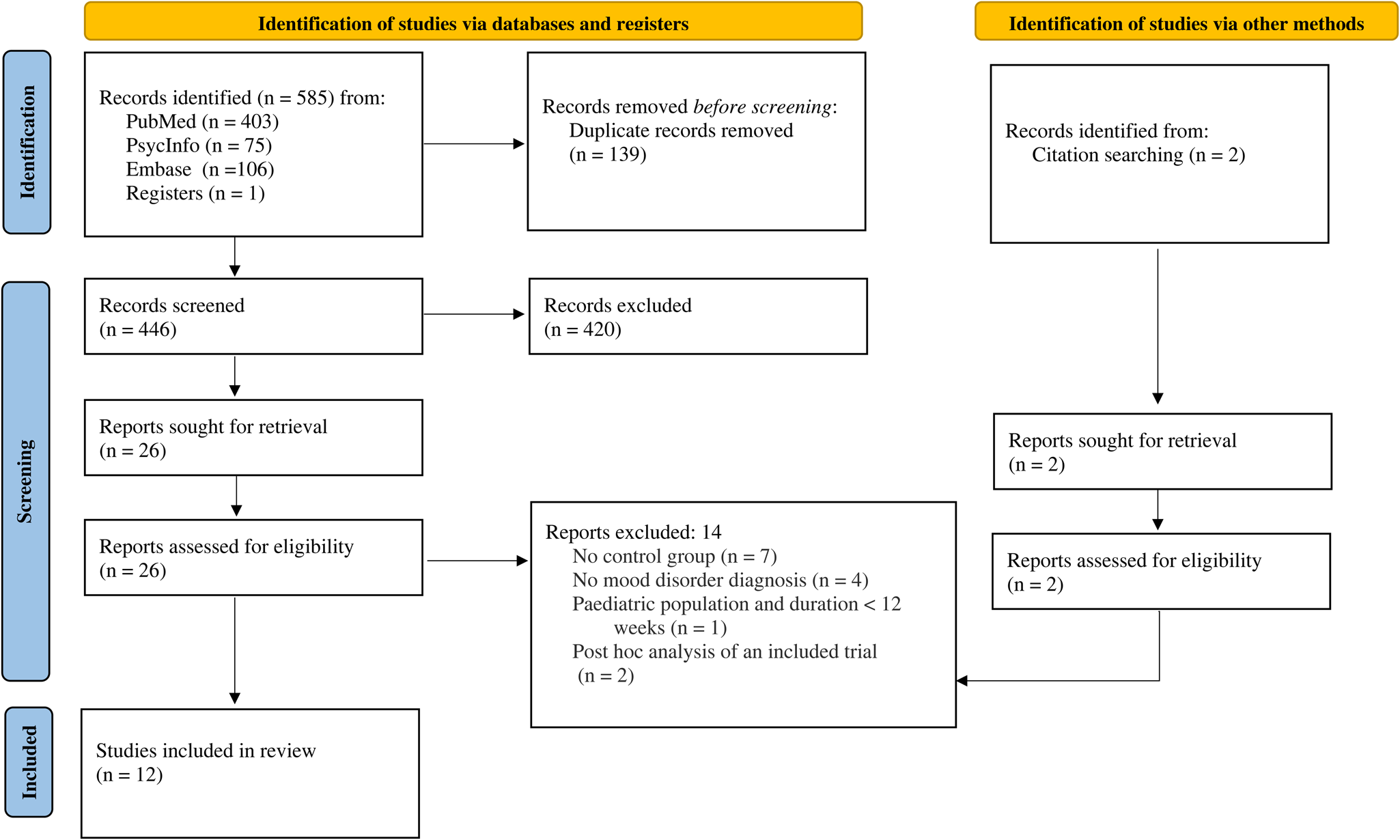
Fig. 1. PRISMA flow diagram.
Quality ratings
Risk of bias assessments are displayed in the online Supplementary Fig. S1. Overall, most trials were judged to be of low risk of bias regarding retention (low attrition), whilst weaknesses concerned lack of detail with reporting randomisation procedures and allocation concealment as well as insufficient masking of treatment allocation. In the two trials in which this was tested and reported, between 65 and 68% of participants randomised to take lithium correctly identified their allocation, whilst rates of correct guessing on placebo were no better than chance (Sackeim et al., Reference Sackeim, Haskett, Mulsant, Thase, Mann, Pettinati, Greenberg, Crowe, Cooper and Prudic2001; Katz et al., Reference Katz, Rogers, Lew, Thwin, Doros, Ahearn, Ostacher, DeLisi, Smith, Ringer, Ferguson, Hoffman, Kaufman, Paik, Conrad, Holmberg, Boney, Huang, Liang and Li+ plus2022). In three trials in which data could be checked against study protocols, there was no evidence of selective reporting.
Analysis of suicide
The main analysis involved all 12 trials, which included 1278 participants randomised to lithium and 1300 randomised to placebo or treatment as usual. Two suicides were identified among people randomised to lithium (0.2% of participants across all included studies) and five among those randomised to placebo or treatment as usual (0.4%). The difference was not statistically significant using Fisher's Exact test; OR = 0.41 (0.03–2.49), p = 0.45. The difference was also not statistically significant when the analysis was restricted to the 11 trials in which suicide was explicitly reported or confirmed with the authors; 0.2 v. 0.4%; OR = 0.41 (0.04–2.49), p = 0.45. We did another sensitivity analysis adding in 15 trials published before 2000, mostly in the 1970s, that were included in the review conducted by Cipriani et al. in 2005 (Cipriani et al., Reference Cipriani, Pretty, Hawton and Geddes2005), updated in 2013 (Cipriani et al., Reference Cipriani, Hawton, Stockton and Geddes2013) and 2017 (Smith and Cipriani, Reference Smith and Cipriani2017). There were two suicides among 1953 people treated with lithium in total (0.10%) and seven among 1784 who received placebo (0.39%). The difference was also not statistically significant using Fisher's Exact test (p = 0.10; OR = 0.26, 95% CI 0.03–1.37) (see online Supplementary Table S4 for details of the pre-2000 trials).
Table 2 displays results from the different meta-analytic methods and Fig. 2 shows the associated Forest plot with results from the Exact and Bayesian methods. The OR using the Exact method was 0.42 (0.01–4.5). None of the methods resulted in statistically significant differences between lithium and placebo or treatment as usual. Sensitivity analysis excluding one trial that did not report suicides also did not show a statistically significant difference (online Supplementary Table S5 and Fig. S2). Sensitivity analysis including the 15 trials from before 2000 also did not show statistically significant effects (the Exact method OR was 0.33, 95% CI 0–3.65) (Fig. 3 and online Supplementary Table S6).
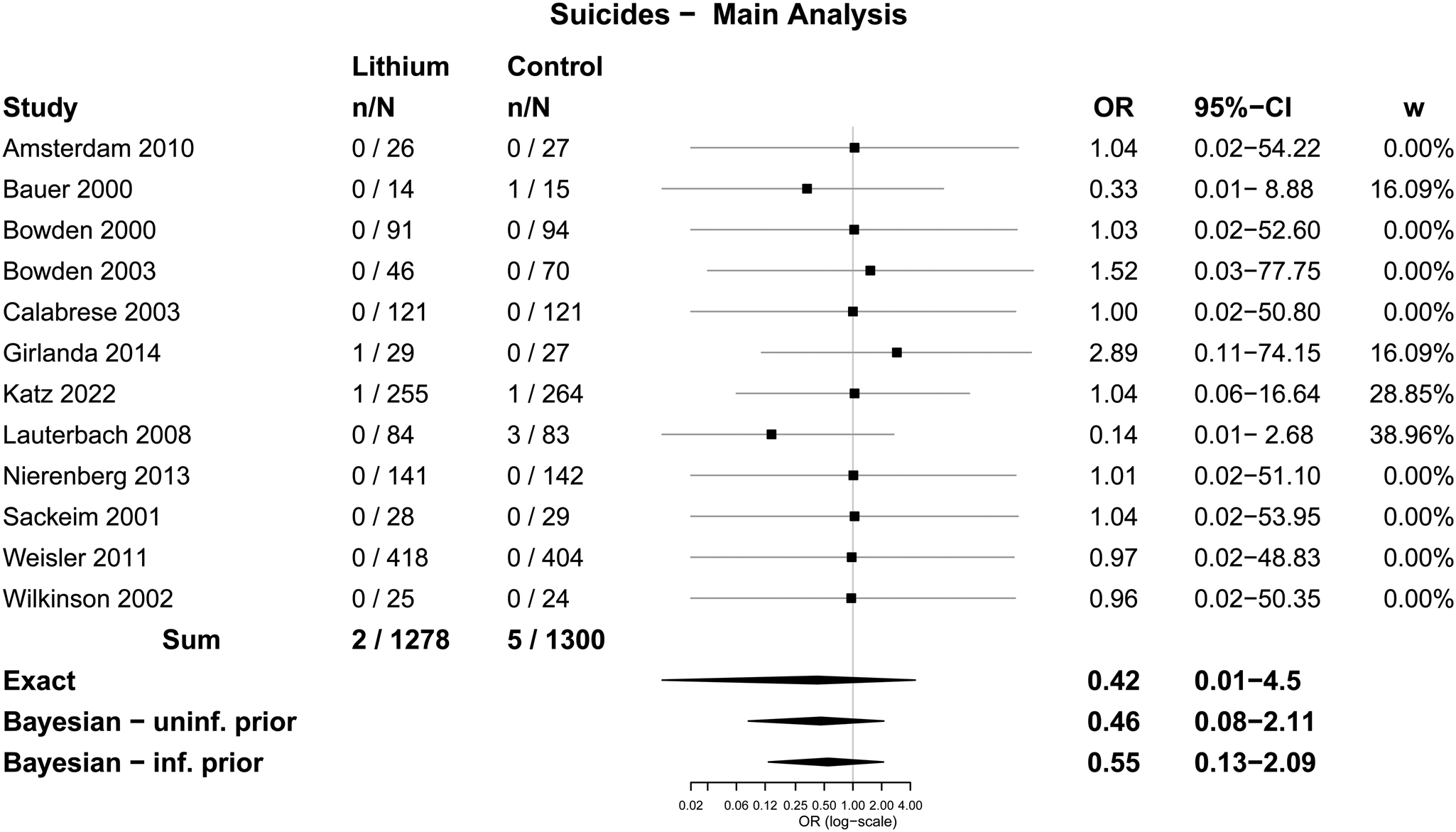
Fig. 2. Forest plot: suicides.

Fig. 3. Forest plot: suicides – sensitivity analysis including pre-2000 trials.
Table 2. Meta-analysis of suicides
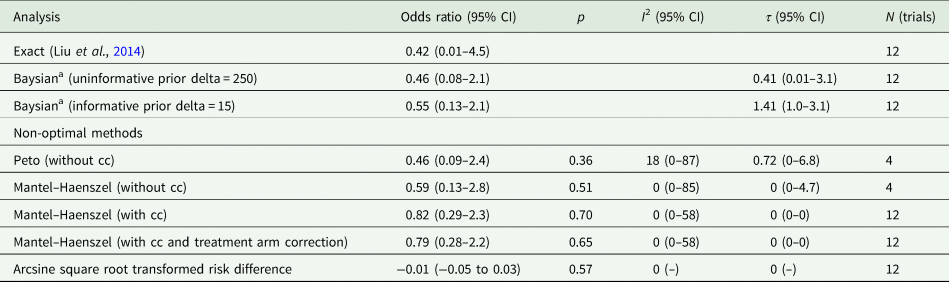
CI, confidence interval; cc, continuity correction; N, number.
a The Bayesian meta-analyses were based on simulations. Because of the special nature of the data (rare events and double zeros), there were occasional large ORs in the posterior after back transforming the log-transformed values of the posterior distribution. The larger ORs skewed the distribution, resulting in slight deviations from sampling to sampling. This affected the upper limits of the credible intervals and the point estimates, but less so the lower limits of the credible intervals. The deviations from sampling to sampling are only minor, affecting mostly the second decimal of the estimations. However, formal analysis provided by the statistical package did not indicate convergence problems. This applies to all the Bayesian analyses performed.
Subgroup analyses involved three trials in which lithium was used specifically to prevent suicide, seven trials involving people who had not taken lithium prior to randomisation, six trials exclusively involving people with bipolar disorders and six trials involving people with depressive disorders or mixed diagnoses. Pooled analysis showed no statistically significant differences in any subgroups (Table 3). Meta-analysis using the Exact method and other methods also showed no statistically significant differences (online Supplementary Tables S7–S10 and Figs S3–S6)
Table 3. Pooled subgroup analyses of suicides in randomised control trials comparing lithium and placebo or treatment as usual

Analysis of non-fatal suicidal behaviour
Seven trials were included in the analysis of suicidal behaviour, which involved a total of 1975 participants. There were 81 people who undertook some form of non-fatal suicidal behaviour among 1278 participants randomised to lithium (6.3%) and 85 among 1300 people randomised to placebo or treatment as usual (6.5%). The difference was not statistically significant using Fisher's Exact test; OR = 0.97 (0.70–1.34), p = 0.87. In five trials that specified suicide attempts, 23 out of 532 people randomised to lithium made a suicide attempt (4.3%), and 21 out of 565 randomised to placebo (3.7%), which was not a statistically significant difference; OR = 1.17 (0.61–2.25), p = 0.65.
Meta-analysis with the Exact method produced an OR of 0.97 (0.68–1.37) for any non-fatal suicidal behaviour, and an OR of 1.13 (0.60–2.14) for suicide attempts. Other methods produced similar results and none showed a statistically significant difference (Table 4, Figs 4 and 5).
Table 4. Meta-analysis of suicidal behaviour and suicide attempts
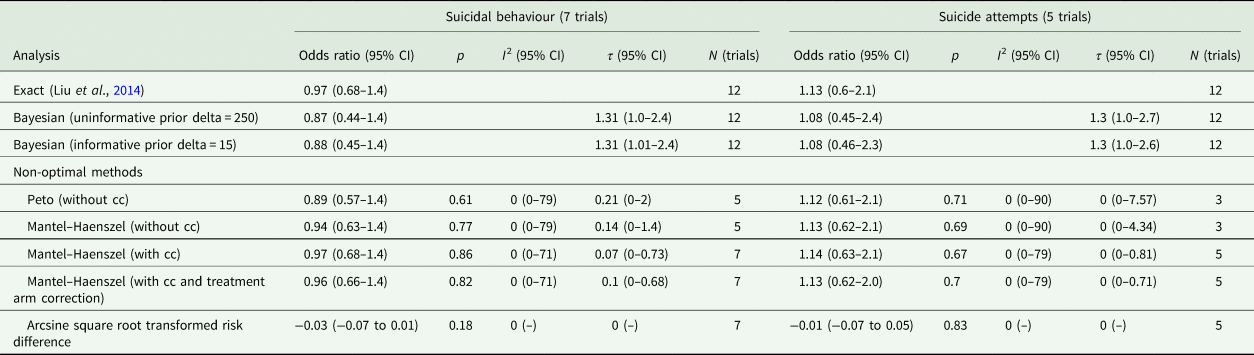
CI, confidence interval; cc, continuity correction; N, number.

Fig. 4. Forest plot: suicidal behaviour.

Fig. 5. Forest plot: suicide attempts.
Discussion
Suicide is a complex phenomenon whose epidemiology and causes vary across cultures (Kirmayer, Reference Kirmayer2022). The WHO recommends a multi-pronged approach to prevention including individual and societal-level interventions (World Health Organisation, 2022). Our analysis shows that the evidence from randomised trials from the new millennium is highly inconclusive and compatible with lithium being associated with an unchanged, decreased or increased risk of suicide. This challenges the results of previous meta-analyses of randomised trials (Cipriani et al., Reference Cipriani, Hawton, Stockton and Geddes2013; Smith and Cipriani, Reference Smith and Cipriani2017), guideline recommendations (Veterans Affairs and Department of Defence, (2019) and the longstanding consensus that lithium reduces the risk of suicide. It contrasts with ecological studies that find associations between lithium levels in drinking water and suicide rates, suggesting they may have been influenced by publication bias, which is evident in some reviews (Eyre-Watt et al., Reference Eyre-Watt, Mahendran, Suetani, Firth, Kisely and Siskind2021). In contrast, our results are consistent with a previous systematic review, although this only included a single trial (Borjesson and Gotzsche, Reference Borjesson and Gotzsche2019), with an earlier Cochrane review published in 2001, which included four trials in total, two of which were included in the meta-analysis (Burgess et al., Reference Burgess, Geddes, Hawton, Townsend, Jamison and Goodwin2001), and with the fact that the largest trial of lithium for suicide prevention, published in 2021, was terminated early due to lack of effect (Katz et al., Reference Katz, Rogers, Lew, Thwin, Doros, Ahearn, Ostacher, DeLisi, Smith, Ringer, Ferguson, Hoffman, Kaufman, Paik, Conrad, Holmberg, Boney, Huang, Liang and Li+ plus2022).
The difference between our results and previous meta-analyses that found statistically significant effects (Cipriani et al., Reference Cipriani, Hawton, Stockton and Geddes2013) or borderline effects (Riblet et al., Reference Riblet, Shiner, Young-Xu and Watts2017) is partly due to the accumulation of further data, and partly to the inclusion of much data that were previously excluded due to the use of the Peto method that excludes trials with zero events. Traditionally, such trials have been included by applying a continuity correction, but this renders results less accurate and is not recommended (Friedrich et al., Reference Friedrich, Adhikari and Beyene2007; Efthimiou, Reference Efthimiou2018). Pooling of data is one way to address this situation and include accurate data from trials with zero events and is frequently employed by drug regulatory bodies, which are concerned with identifying rare events (Lievre et al., Reference Lievre, Cucherat and Leizorovicz2002; Bradburn et al., Reference Bradburn, Deeks, Berlin and Russell Localio2007). The Exact method and Bayesian methods of meta-analysis, which include data from trials with zero events, have also been recommended recently (Ren et al., Reference Ren, Lin, Lian, Zou and Chu2019). Thus, our analysis of suicide is based on 12 trials and 2578 participants in total, whereas the previous most influential analysis included only four trials with 485 participants in total (Cipriani et al., Reference Cipriani, Hawton, Stockton and Geddes2013)
Another reason for the variation between our findings and prior reviews is that we only included trials published after 2000. We took this decision because reporting standards before 2000 were not as rigorous and therefore we cannot be certain that trials published before this date reliably reported adverse events, including suicides, especially since no studies prior to this date were set up to test for suicide prevention effects specifically. In fact, we know that a suicide was not reported in at least one pre-2000 study paper (Glen et al., Reference Glen, Johnson and Shepherd1984) (among people randomised to amitriptyline) from data obtained subsequently by Cipriani et al. (Reference Cipriani, Hawton, Stockton and Geddes2013). Ioannidis and Lau (49) examined 192 drug trials published between 1967 and 1999 and found that only 39% adequately reported adverse events. Since suicide is rare, the omission of even one or two events may significantly influence the analysis. Following the publication and widespread adoption of the CONSORT statement guidelines in the late 1990s (Begg et al., Reference Begg, Cho, Eastwood, Horton, Moher, Olkin, Pitkin, Rennie, Schulz, Simel and Stroup1996), we can be more confident that trials would reliably report serious adverse events such as suicide. Our sensitivity analysis including trials published before 2000 revealed two further suicides in people allocated to placebo, but there remained no evidence of a statistically significant difference between lithium and the control condition.
Results of previous analyses have also been influenced by three suicides that occurred in the placebo group in one study, the suicide prevention study by Lauterbach et al. (Reference Lauterbach, Felber, Muller-Oerlinghausen, Ahrens, Bronisch, Meyer, Kilb, Lewitzka, Hawellek, Quante, Richter, Broocks and Hohagen2008). This study was reported as being double-blind, but the authors describe how the blind was broken in cases where participants were suspected of non-adherence due to low levels of blood lithium, or when there was a suspected risk of suicide. The former situation is likely to have been common since mean lithium levels were below the intended therapeutic window for the majority of the trial (Lauterbach et al., Reference Lauterbach, Felber, Muller-Oerlinghausen, Ahrens, Bronisch, Meyer, Kilb, Lewitzka, Hawellek, Quante, Richter, Broocks and Hohagen2008, p. 474). It is plausible, therefore, that the increased monitoring instituted in unblinded participants to maintain adherence or reduce suicide risk might have resulted in fewer suicides in the lithium group. This interpretation is supported by research that shows that greater access to clinical care and closer monitoring can reduce the risk of suicide in clinical and general populations (Tondo et al., Reference Tondo, Albert and Baldessarini2006; Sakinofsky, Reference Sakinofsky2014).
Risk of bias assessments revealed that most included studies had strengths, such as low attrition, and blinding of assessments. Blinding of participants was judged to be insecure across all trials, however, due to the likelihood of the side effects of lithium revealing the identity of medication. There was no evidence of selective reporting, but this was only possible to check in a minority of trials. The risk of bias tool used did not assess aspects of quality that are particularly relevant to rare outcomes, such as sample size and the quality of reporting of adverse events.
We made several protocol changes in order to make the analysis more robust and comprehensive. Thus we searched some trial registers and we added meta-analysis using different methods suggested recently in the statistical literature conducting all these using R instead of Revman. This allowed us to perform a more sophisticated analysis, and we have made the R code publicly available. We also added a sensitivity analysis including trials published before 2000, in order to compare our findings with those of previous reviews. We changed the terminology for describing our secondary outcome from ‘self-harm’ to ‘suicidal behaviour’, since this reflected the language used in most of the included studies and has a clearer relationship to actual suicide because suicide attempts are defined as actions with the intent to die. We also performed an additional analysis of suicide attempts specifically. Another limitation is that full-text screening was not completed independently by two reviewers for the whole sample.
Traditionally, pooling data are criticised because it neglects between-study heterogeneity and estimates may therefore not be sufficiently conservative. However, no statistically significant effect of lithium was found for any of the outcomes we examined using this method, which was consistent with results produced by the different methods of meta-analysis. Heterogeneity of studies could only be estimated with much imprecision. Subgroup analyses did not identify any obvious sources of variation, such as whether the trial included people at high risk of suicide (as in the trials designed to study suicide prevention specifically), the diagnoses of participants and prior treatment with lithium. However, these analyses were based on small numbers of participants and trials and should be interpreted cautiously.
Another review found similar results to our own, but this review not only excluded studies with zero events, but also studies in which participants had been taking lithium prior to study entry. Thus the analysis of suicide was based on a single study (Lauterbach et al., Reference Lauterbach, Felber, Muller-Oerlinghausen, Ahrens, Bronisch, Meyer, Kilb, Lewitzka, Hawellek, Quante, Richter, Broocks and Hohagen2008), involving 167 participants (Borjesson and Gotzsche, Reference Borjesson and Gotzsche2019). The justification for excluding studies in which participants were taking lithium prior to randomisation was that all psychoactive drugs produce withdrawal syndromes, which may heighten suicide risk (Baldessarini and Tondo, Reference Baldessarini and Tondo2019; Cohen and Recalt, Reference Cohen and Recalt2019). One empirical study does indicate that suicidal acts were more common in the year following lithium discontinuation than before lithium was started (Baldessarini et al., Reference Baldessarini, Tondo and Hennen1999). In addition, lithium discontinuation is associated with an increased risk of relapse of bipolar disorder above baseline (Suppes et al., Reference Suppes, Baldessarini, Faedda and Tohen1991). In our analysis, one of the included studies reported that suicidal behaviour was more common among participants who stopped their randomised treatment prematurely, but this applied to those who were randomised to both lithium and placebo (Katz et al., Reference Katz, Rogers, Lew, Thwin, Doros, Ahearn, Ostacher, DeLisi, Smith, Ringer, Ferguson, Hoffman, Kaufman, Paik, Conrad, Holmberg, Boney, Huang, Liang and Li+ plus2022). Our subgroup analysis of trials in which participants had not been treated with lithium previously did not find a difference between lithium and placebo, but the analysis lacked statistical power.
Conclusion
The evidence from randomised trials of the new millennium is inconclusive and does not provide support for the belief that lithium reduces the risk of suicide or suicidal behaviour. More data are needed to estimate the effect of lithium with more precision in general, and in subgroups of patients, specifically.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S204579602200049X
Data
All data are available in the published paper and the Supplementary material. The R code used for meta-analysis is available via the Open Science Framework at https://osf.io/6q3w7/
Acknowledgements
The authors would like to thank Klaus Munkholm for providing useful comments on a draft of the manuscript
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of interest
JM is co-chair person of the Critical Psychiatry Network, a board member of the Council for Evidence-based Psychiatry and receives modest royalties from books about psychiatric drugs. No other authors have any conflicts of interest.
Ethical standards
Ethical approval was not required.













