Introduction
Major depression is among the leading causes of burden of disease worldwide (GBD 2017 Disease and Injury Incidence and Prevalence Collaborators, 2017). It is not only one of the most common and most detrimental mental disorders affecting psychosocial functioning but also known as a risk factor for various other outcomes such as dementia (Kaup et al., Reference Kaup, Byers, Falvey, Simonsick, Satterfield, Ayonayon, Smagula, Rubin and Yaffe2016). The lifetime prevalence of depression was estimated at 6–21% (Bromet et al., Reference Bromet, Andrade, Hwang, Sampson, Alonso, de Girolamo, de Graaf, Demyttenaere, Hu, Iwata, Karam, Kaur, Kostyuchenko, Lépine, Levinson, Matschinger, Mora, Browne, Posada-Villa, Viana, Williams and Kessler2011), implying that one in five people experience depression at some point in their life. The years lived with disability attributed to depression has increased, accounting for 8% of global health loss in 2010 (Vos et al., Reference Vos, Flaxman, Naghavi, Lozano, Michaud, Ezzati, Shibuya, Salomon, Abdalla, Aboyans, Abraham, Ackerman, Aggarwal, Ahn, Ali, Alvarado, Anderson, Anderson, Andrews, Atkinson, Baddour, Bahalim, Barker-Collo, Barrero, Bartels, Basáñez, Baxter, Bell, Benjamin, Bennett, Bernabé, Bhalla, Bhandari, Bikbov, Abdulhak, Birbeck, Black, Blencowe, Blore, Blyth, Bolliger, Bonaventure, Boufous, Bourne, Boussinesq, Braithwaite, Brayne, Bridgett, Brooker, Brooks, Brugha, Bryan-Hancock, Bucello, Buchbinder, Buckle, Budke, Burch, Burney, Burstein, Calabria, Campbell, Canter, Carabin, Carapetis, Carmona, Cella, Charlson, Chen, Cheng, Chou, Chugh, Coffeng, Colan, Colquhoun, Colson, Condon, Connor, Cooper, Corriere, Cortinovis, De Vaccaro, Couser, Cowie, Criqui, Cross, Dabhadkar, Dahiya, Dahodwala, Damsere-Derry, Danaei, Davis, De Leo, Degenhardt, Dellavalle, Delossantos, Denenberg, Derrett, Des Jarlais, Dharmaratne, Dherani, Diaz-Torne, Dolk, Dorsey, Driscoll, Duber, Ebel, Edmond, Elbaz, Ali, Erskine, Erwin, Espindola, Ewoigbokhan, Farzadfar, Feigin, Felson, Ferrari, Ferri, Fèvre, Finucane, Flaxman, Flood, Foreman, Forouzanfar, Fowkes, Franklin, Fransen, Freeman, Gabbe, Gabriel, Gakidou, Ganatra, Garcia, Gaspari, Gillum, Gmel, Gonzalez-Medina, Gosselin, Grainger, Grant, Groeger, Guillemin, Gunnell, Gupta, Haagsma, Hagan, Halasa, Hall, Haring, Haro, Harrison, Havmoeller, Hay, Higashi, Hill, Hoen, Hoffman, Hotez, Hoy, Huang, Ibeanusi, Jacobsen, James, Jarvis, Jasrasaria, Jayaraman, Johns, Jonas, Karthikeyan, Kassebaum, Kawakami, Keren, Khoo, King, Knowlton, Kobusingye, Koranteng, Krishnamurthi, Laden, Lalloo, Laslett, Lathlean, Leasher, Lee, Leigh, Levinson, Lim, Limb, Lin, Lipnick, Lipshultz, Liu, Loane, Ohno, Lyons, Ma, Mabweijano, MacIntyre, Malekzadeh, Mallinger, Manivannan, Marcenes, March, Margolis, Marks, Marks, Matsumori, Matzopoulos, Mayosi, McAnulty, McDermott, McGill, McGrath, Medina-Mora, Meltzer, Mensah, Merriman, Meyer, Miglioli, Miller, Miller, Mitchell, Mock, Mocumbi, Moffitt, Mokdad, Monasta, Montico, Moran, Morawska, Mori, Murdoch, Mwaniki, Naidoo, Nair, Naldi, Narayan, Nelson, Nelson, Nevitt, Newton, Nolte, Norman, Norman, O'Donnell, O'Hanlon, Olives, Omer, Ortblad, Osborne, Ozgediz, Page, Pahari, Pandian, Rivero, Patten, Pearce, Padilla, Perez-Ruiz, Perico, Pesudovs, Phillips, Phillips, Pierce, Pion, Polanczyk, Polinder, Pope, Popova, Porrini, Pourmalek, Prince, Pullan, Ramaiah, Ranganathan, Razavi, Regan, Rehm, Rein, Remuzzi, Richardson, Rivara, Roberts, Robinson, De Leòn, Ronfani, Room, Rosenfeld, Rushton, Sacco, Saha, Sampson, Sanchez-Riera, Sanman, Schwebel, Scott, Segui-Gomez, Shahraz, Shepard, Shin, Shivakoti, Singh, Singh, Singh, Singleton, Sleet, Sliwa, Smith, Smith, Stapelberg, Steer, Steiner, Stolk, Stovner, Sudfeld, Syed, Tamburlini, Tavakkoli, Taylor, Taylor, Taylor, Thomas, Thomson, Thurston, Tleyjeh, Tonelli, Towbin, Truelsen, Tsilimbaris, Ubeda, Undurraga, Van Der Werf, Van Os, Vavilala, Venketasubramanian, Wang, Wang, Watt, Weatherall, Weinstock, Weintraub, Weisskopf, Weissman, White, Whiteford, Wiebe, Wiersma, Wilkinson, Williams, Williams, Witt, Wolfe, Woolf, Wulf, Yeh, Zaidi, Zheng, Zonies, Lopez, Murray and Moradi-Lakeh2012).
Oral diseases are another major public health issue affecting more than 3.5 billion people worldwide and imply the third highest treatment costs after diabetes and cardiovascular diseases (Listl et al., Reference Listl, Galloway, Mossey and Marcenes2015; Peres et al., Reference Peres, Macpherson, Weyant, Daly, Venturelli, Mathur, Listl, Celeste, Guarnizo-Herreño, Kearns, Benzian, Allison and Watt2019). Despite its critical relevance for people's quality of life and well-being, the prevention and management of oral diseases has been subject to woeful neglect and significant policy failures (Watt et al., Reference Watt, Daly, Allison, Macpherson, Venturelli, Listl, Weyant, Mathur, Guarnizo-Herreño, Celeste, Peres, Kearns and Benzian2019). One of the main reasons for such neglect could be the previous absence of causal evidence for links between oral health and other primary relevant health outcomes, particularly mental health. Oral conditions have previously been reported to be associated with people's mental health and well-being but, so far, there is no causal evidence for links between oral conditions and mental health outcomes (Cademartori et al., Reference Cademartori, Gastal, Nascimento, Demarco and Corrêa2018). Deciphering the mechanisms between oral and mental health outcomes is not only relevant to better target prevention and treatment of these conditions but also critical for global health advocacy to adequately prioritise mental and oral health on the global health policy agenda. Potential causal links between oral and mental health conditions must be critically scrutinised.
More specifically, there is no causal evidence on the impact of teeth on depression. It has been hypothesised that neuroinflammation due to past periodontal inflammation or autonomic nerve imbalance due to oral-related pain, stress and discomfort increases depressive symptoms; thereby, tooth loss is expected to increase depression (Cademartori et al., Reference Cademartori, Gastal, Nascimento, Demarco and Corrêa2018). Declines in social functioning due to poor oral health (Rouxel et al., Reference Rouxel, Heilmann, Demakakos, Aida, Tsakos and Watt2017) might also explain such associations.
The main shortcomings in previous literature about the effect of oral conditions on depression relate to the possibility of reverse causation or other confounding bias. The instrumental variable (IV) approach is a quasi-experimental method to estimate causal effects in observational data. It is a potentially powerful tool, especially when a randomised controlled trial (RCT) is not feasible due to ethical concerns or practical difficulties (Angrist and Krueger, Reference Angrist and Krueger2001; Maciejewski and Brookhart, Reference Maciejewski and Brookhart2019). Studies on the association between oral health and depression are typical cases, as both conditions take a long course and are thus difficult to be followed up. The IV approach emulates an RCT by employing exogenous, quasi-randomly assigned variation in a so-called IV – or instrument – that drives the probability or intensity of an exposure or treatment. Under the assumption that the IV has no direct effect on the outcome, the IV method identifies a causal effect even in the presence of unobserved confounders.
Water fluoridation is an evidence-based, robust measure for caries prevention (McDonagh et al., Reference McDonagh, Whiting, Wilson, Sutton, Chestnutt, Cooper, Misso, Bradley, Treasure and Kleijnen2000), and the benefit continues for tooth loss in adulthood (Neidell et al., Reference Neidell, Herzog and Glied2010). Adverse effects of water fluoridation on other outcomes such as cognitive ability have not been observed in many reviews (McDonagh et al., Reference McDonagh, Whiting, Wilson, Sutton, Chestnutt, Cooper, Misso, Bradley, Treasure and Kleijnen2000). Accordingly, fluoride utilisation is a candidate instrument to examine the causal effect of tooth loss on depression. In the USA, water fluoridation has been implemented since 1945, and the year of introduction and the proportion of people supplied fluoridated water varied by county (eFig. 1 in the Supplementary Material) (US Department of Health and Human Services, 1993). This natural experiment induced historical and geographical variation in fluoride exposure in the US population. By employing this exogenous difference, we scrutinised the causal effect of tooth loss on depression in US adults.
Method
Data
The data of the cross-sectional survey of the Behavioral Risk Factor Surveillance System (BRFSS) waves 2006, 2008 and 2010 were pooled and analysed. The BRFSS is a state-based telephone survey of the population aged 18 years or older in the USA (Centers for Disease Control and Prevention, 2010). The median state response rate was 51.4, 53.3 and 54.6% in 2006, 2008 and 2010, respectively (Centers for Disease Control and Prevention, 2010). We used all waves and states that contain information on the number of lost teeth, depression and county of residence. The analytical sample was restricted to respondents born in 1940–1978 because information on water fluoridation during youth was available only for these cohorts. Further excluding the respondents with missing data, 169 061 respondents’ data were included in the analysis (eFig. 2 in the Supplementary Material). This study was approved by the ethical committee at Tokyo Medical and Dental University. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Instrumental variable
Our instrument was the exogenous variation in water fluoridation by county in the USA, namely the geographical and historical difference in the proportion of people supplied with fluoridated water. County-level water fluoridation is a suitable instrument because it affects the number of lost teeth in the residents through its preventive effect on dental caries (McDonagh et al., Reference McDonagh, Whiting, Wilson, Sutton, Chestnutt, Cooper, Misso, Bradley, Treasure and Kleijnen2000), while it does not influence the incidence of depression directly and it is arguably not correlated with unobserved determinants of depression.
The information on water fluoridation was obtained from the 1992 Water Fluoridation Census (US Department of Health and Human Services, 1993), which reported the number of the population supplied with fluoridated water by counties every year from 1945 to 1992. We followed a previous study (Glied and Neidell, Reference Glied and Neidell2010) and accumulated the county-level proportion of people supplied fluoridated water across years when respondents were 5–14 years old, which corresponds to post-eruptive enamel maturation of permanent teeth (i.e. sensitive for benefit from fluoride). The total population was fixed to the 1990s estimate. The variable was rescaled between 0 and 1 in the analysis so that the coefficient indicates the difference between no exposure and full exposure of water fluoride.
Treatment variable
The treatment variable, the number of lost teeth, was assessed by a single question. ‘How many of your permanent teeth have been removed because of tooth decay or gum disease? Include teeth lost to infection, but do not include teeth lost for other reasons, such as injury or orthodontics.’ with response options of ‘None’, ‘1 to 5’, ‘6 or more but not all’ and ‘All’. We did not consider wisdom teeth and assigned the mid-point for each category (i.e. 0, 3, 16.5 and 28, respectively) to construct a continuous variable for the number of lost teeth in the main analysis. Analysis with a dichotomised variable (⩾1 tooth lost v. full dentition; and lost all teeth v. having ⩾1 tooth) was also performed.
Outcome variable
The outcome, depression, was assessed by the eight-item version of the Patient Health Questionnaire (PHQ-8) (Kroenke et al., Reference Kroenke, Spitzer and Williams2001). It consists of eight questions on the frequency that the respondent experienced particular depressive symptoms over the past 2 weeks. In the BRFSS, participants were asked the number of days that they experienced the eight symptoms. The responses were converted to a score between ‘not at all’ (0 points) and ‘nearly every day’ (3 points) (see eMethod 1) (Kroenke et al., Reference Kroenke, Spitzer and Williams2001). The total score (range 0–24) as a continuous variable and a binary variable indicating probable major depression (PHQ-8 ⩾ 10) (Wu et al., Reference Wu, Levis, Riehm, Saadat, Levis, Azar, Rice, Boruff, Cuijpers, Gilbody, Ioannidis, Kloda, Mcmillan, Patten, Shrier, Ziegelstein, Akena, Arroll, Ayalon, Baradaran, Baron, Bombardier, Butterworth, Carter, Chagas, Chan, Cholera, Conwell, De Man-Van Ginkel, Fann, Fischer, Fung, Gelaye, Goodyear-Smith, Greeno, Hall, Harrison, Härter, Hegerl, Hides, Hobfoll, Hudson, Hyphantis, Inagaki, Jetté, Khamseh, Kiely, Kwan, Lamers, Liu, Lotrakul, Loureiro, Löwe, Mcguire, Mohd-Sidik, Munhoz, Muramatsu, Osório, Patel, Pence, Persoons, Picardi, Reuter, Rooney, Santos, Shaaban, Sidebottom, Simning, Stafford, Sung, Tan, Turner, Van Weert, White, Whooley, Winkley, Yamada, Benedetti and Thombs2019) were used as outcome variables.
Analysis
We used a two-stage least squares (2SLS) regression analysis to identify the causal effect of tooth loss on depression. The first stage was a regression of the treatment variable (number of lost teeth) on the instrument (fluoride exposure), adjusting for year of birth, wave of the survey, gender and state of residence. In the second-stage regression, the outcome (depression) was regressed on the predicted number of lost teeth adjusting for the same covariates. Thereby, the second-stage coefficient indicates the causal effect of tooth loss on depression. Ordinary Least Squares (OLS) estimation was also performed to compare the effect sizes because the 2SLS estimate can lead to a greater bias than OLS when the instrument is only weakly correlated with the treatment variable (Angrist and Krueger, Reference Angrist and Krueger2001).
To evaluate whether the effect of tooth loss varies by population characteristics, analyses stratified by age, year of birth, gender, median household income and dental care utilisation were performed. We also performed a sensitivity analysis by assigning mean, median and mode of the clinically examined number of lost teeth reported in a previous study (Sekundo et al., Reference Sekundo, Stock, Jürges and Listl2019) for the brackets of self-reported 1–5 or 6–27 lost teeth.
Results
Table 1 describes demographic characteristics of the 169 061 respondents (mean age = 50.0; range of age = 28–70; male = 38.7%). The average number of lost teeth was 4.5 when assigning the mid-point to each category. People losing more teeth reported higher depression symptoms while less likely to have been exposed to fluoridated water in childhood.
Table 1. Demographic characteristics of respondents; N = 169 061a
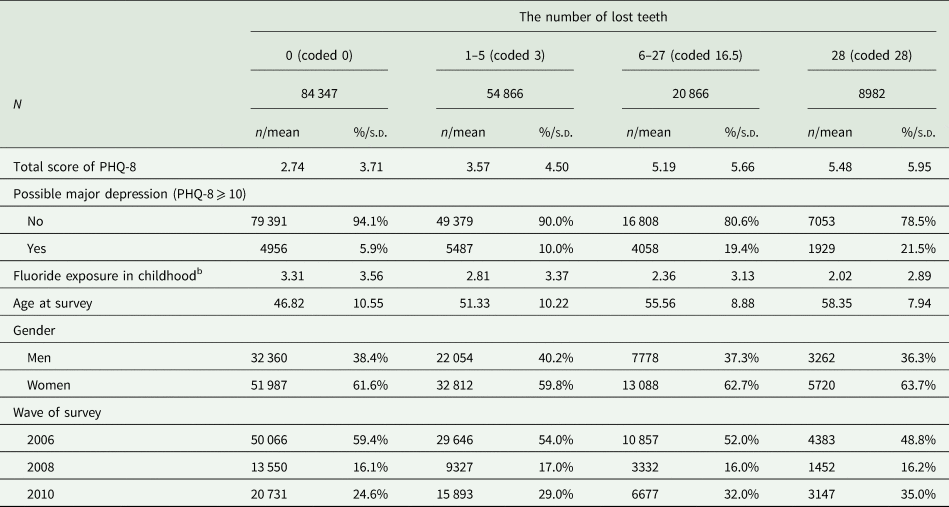
PHQ-8, the eight-item Patient Health Questionnaire depression scale; s.d., standard deviation.
a 36 states were included: Alabama, Arizona, Arkansas, California, Delaware, District of Columbia, Florida, Georgia, Hawaii, Idaho, Illinois, Indiana, Iowa, Louisiana, Maine, Michigan, Minnesota, Mississippi, Missouri, Montana, Nevada, New Hampshire, New Mexico, North Dakota, Oklahoma, Oregon, Rhode Island, South Carolina, Tennessee, Texas, Utah, Vermont, Virginia, West Virginia, Wisconsin and Wyoming.
b Sum of the proportion of people supplied with fluoridated water in the county of residence during the age of 5–14 years.
Table 2 shows the causal effect of the number of lost teeth on depression symptoms (i.e. total score of PHQ-8). The first-stage regression showed that exposure to fluoridated water prevented losing 0.564 teeth [95% confidence interval (CI) 0.435–0.693; F-statistic: 73.4]. The reduced form equation showed a negative overall effect of being exposed to fluoridated water on depression symptoms. Under the assumption that fluoride exposure affects depression only via dental health, the second-stage regression showed a causal effect of tooth loss on depression: losing a tooth increased depression symptom by 0.146 PHQ-8 total score points (95% CI 0.008–0.284). The effect size was close to the OLS estimate (coefficient = 0.134; 95% CI 0.131–0.136). When tooth loss was dichotomised, having lost ⩾1 tooth and having lost all teeth also increased depressive symptoms [coefficients (95% CI) are 3.141 (0.100–6.182) and 7.251 (0.053–14.449), respectively].
Table 2. Causal effect of tooth loss on a total score of PHQ-8 (ranges 0–24); N = 169 061
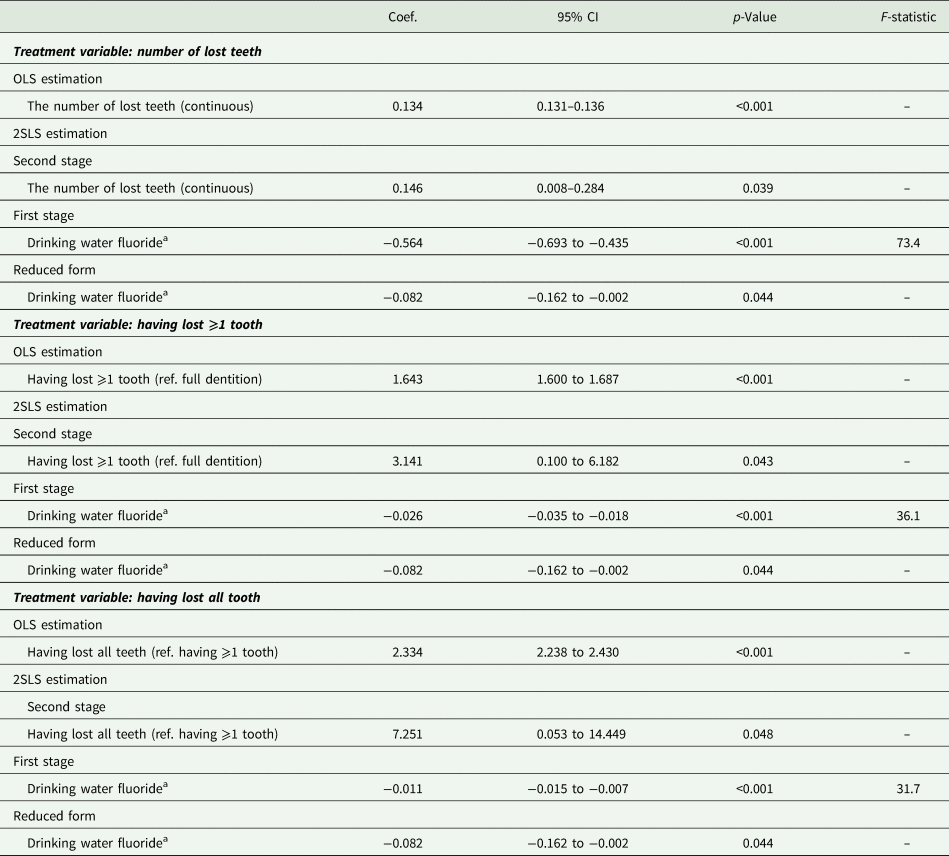
CI, confidence interval; OLS, Ordinary Least Squares; PHQ-8, the eight-item Patient Health Questionnaire depression scale; 2SLS, two-stage least-squares.
Adjusted for year of birth, wave of survey, gender and state of residence.
a Sum of the proportion of people supplied with fluoridated water in the county of residence during the age of 5–14 years (rescaled between 0 and 1).
Table 3 shows the effect of the number of lost teeth on probable major depression (i.e. PHQ-8 ⩾ 10). The second-stage regression showed that the probability of major depression increased by 0.81 percentage points (95% CI −0.12 to 1.73) with an additional loss of one tooth. The effect size was slightly larger than the OLS estimate (coefficient = 0.73 percentage points; 95% CI 0.71–0.75). When tooth loss was dichotomised, the probability of major depression increased by tooth loss [coefficients (95% CI) are 17.39 (−2.89 to 37.66) and 40.13 (−7.32 to 87.59) percentage points for having lost ⩾1 tooth and having lost all teeth, respectively].
Table 3. Causal effect of tooth loss on possible major depression (PHQ-8 ⩾ 10); N = 169 061
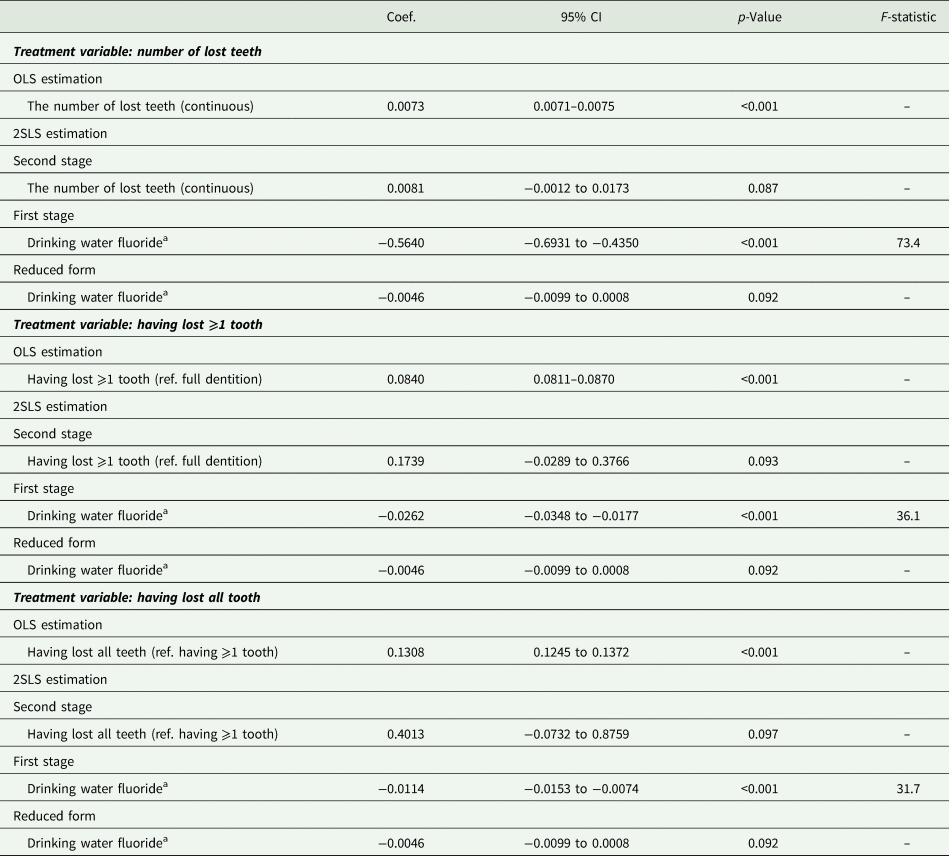
CI, confidence interval; OLS, Ordinary Least Squares; PHQ-8, the eight-item Patient Health Questionnaire depression scale; 2SLS, two-stage least-squares.
Adjusted for year of birth, wave of survey, gender and state of residence.
aSum of the proportion of people supplied with fluoridated water in the county of residence during the age of 5–14 years (rescaled between 0 and 1).
The results from the stratified analyses are illustrated in Fig. 1. The effect of tooth loss on depression symptoms appeared to be more pronounced in younger age groups than in older people [coefficients (95% CI) are 0.182 (0.004–0.361) and−0.055 (−1.019 to 0.909), respectively]. The effect sizes did not differ by year of birth. The point estimate for the effect of tooth loss was larger in men than in women [coefficients (95% CI) are 0.225 (0.027–0.422), 0.094 (−0.100 to 0.287), respectively]. When stratified by annual household income, the preventive effect of fluoridated water on teeth was larger for lower-income groups. The point estimate for the effect of tooth loss was larger in the higher-income group than in the lower-income group [coefficients (95% CI) are 0.257 (0.018–0.496) and 0.080 (−0.102 to 0.262), respectively]. The preventive effect of fluoride exposure on teeth was larger among people with less dental care utilisation. The point estimate for the effect of tooth loss was slightly larger in people less using dental care than those who visit dental clinics more [coefficients (95% CI) are 0.110 (−0.058 to 0.279) and 0.049 (−0.242 to 0.341), respectively].
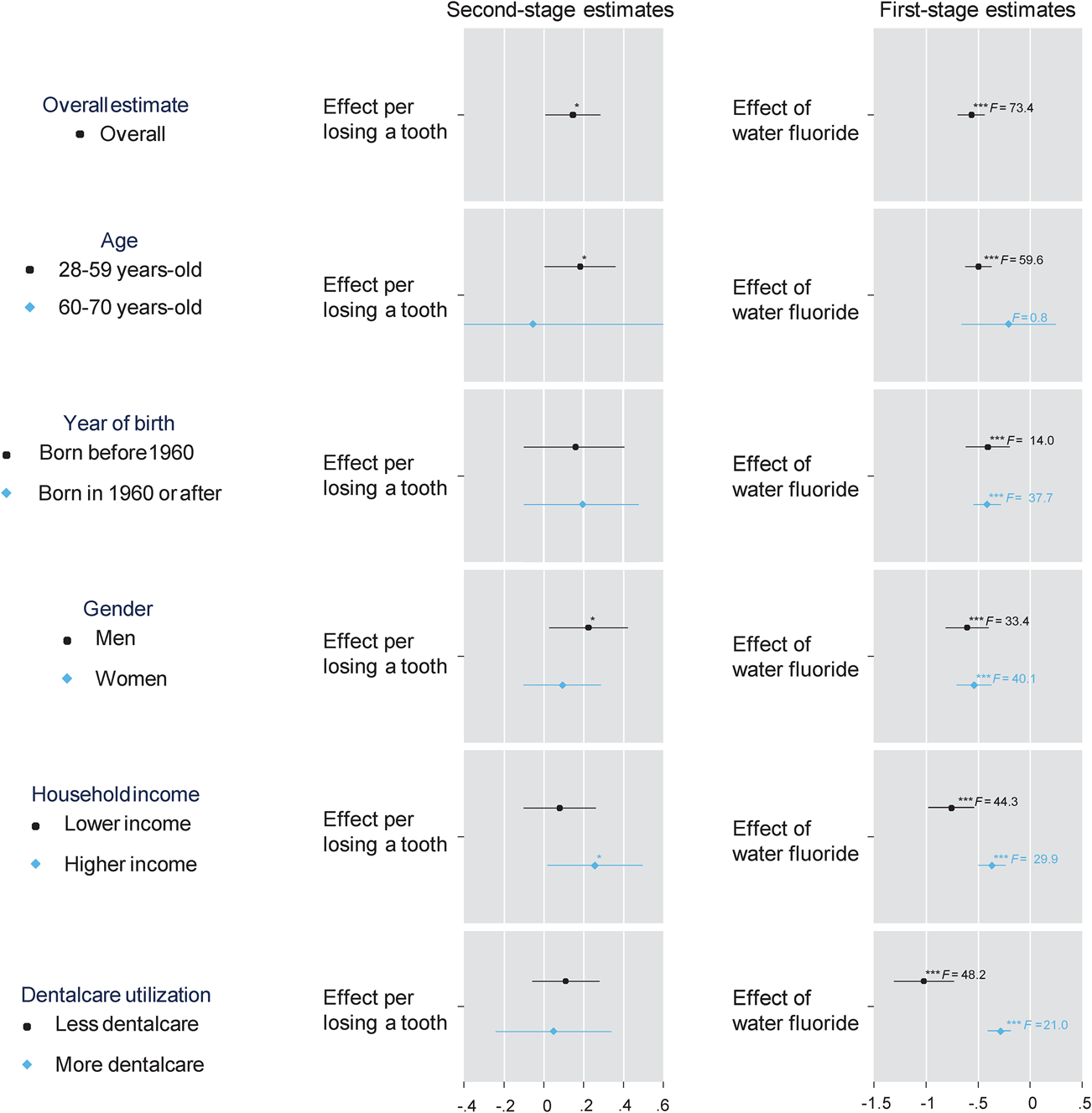
Fig. 1. Causal effect of tooth loss on a total score of the eight-item Patient Health Questionnaire depression scale (PHQ-8) with various stratifications; adjusted for year of birth, wave of survey, gender and state of residence; annual household income was divided at the median (<$50 000 or ⩾$50 000); dental care utilization was divided by having visited dentist within one year or not); the left and right panels illustrate the second- and first-stage estimates, respectively; *p < 0.05, **p < 0.01, ***p < 0.001.
The sensitivity analysis varying the values assigned to the category of tooth loss did not largely change the results, although the second-stage estimate was larger when smaller numbers of lost teeth were assigned (eFig. 3 in the Supplementary Material).
Discussion
To our knowledge, the present study is the first to identify causal effects of tooth loss on depression, whereby reverse causation and the bias due to potential confounding were eliminated by employing an IV approach. An additional loss of one tooth causally increased depression symptoms by 0.146 points or increased the probability of major depression by 0.81 percentage points. For further interpretation of our findings, we compared our estimates with results from RCTs on antidepressant drugs (Cipriani et al., Reference Cipriani, Furukawa, Salanti, Chaimani, Atkinson, Ogawa, Leucht, Ruhe, Turner, Higgins, Egger, Takeshima, Hayasaka, Imai, Shinohara, Tajika, Ioannidis and Geddes2018). The effect size of losing ⩾10 teeth was comparable to adults with major depressive disorder not receiving antidepressant drugs (see eMethod 2).
The effect of teeth on depression seemed to be greater in young adults, men, people with higher income and those with less dental care utilisation. Older people may consider tooth loss as a natural change with ageing and have adapted to it (MacEntee et al., Reference MacEntee, Hole and Stolar1997), thus the psychological impact of tooth loss might be weaker for them. The gender difference is in contrast to women perceiving oral health to be generally more relevant than men (Mason et al., Reference Mason, Pearce, Walls, Parker and Steele2006). In the USA, the prevalence of edentulousness is higher in women than men across most age groups (Slade et al., Reference Slade, Akinkugbe and Sanders2014), suggesting that women are more likely to lose their teeth early. Hence women might be on a different tooth loss trajectory and have adapted to fewer teeth at earlier life stages than men. This might potentially explain some differences in the observed effects of tooth loss for women and men of similar age.
The first-stage regressions stratified by income confirmed the previous literature showing larger benefits of water fluoridation for marginalised people (Riley et al., Reference Riley, Lennon and Ellwood1999). Similar to previous literature on the marginal return to general health (Grossman, Reference Grossman1972), the larger effect size in the second-stage regressions for the higher-income group might reflect a larger marginal return to oral health for high-income groups. If dental care can partially mitigate the deteriorative effects of tooth loss on depression, a limited affordability of dental care would not only be detrimental to oral health but also exacerbate the suffering from depression in low-income groups.
Depression affects more than 320 million people, or 4.4% of the global population (World Health Organization, 2017); while 2.3% of the global population are edentate (Kassebaum et al., Reference Kassebaum, Bernabé, Dahiya, Bhandari, Murray and Marcenes2014). If we could prevent one tooth loss on average, the expected reduction in depression (0.81 percentage points) would account for 60 million people worldwide. We acknowledge this extrapolation might be an oversimplification; however, the present study supports that promoting oral health significantly improves psychological well-being of the global population. Importantly, we identified causal effects from observational data using quasi-randomisation; therefore, the estimated impact on health is not biased by omitted variables or reverse causation. The IV analysis estimates the local average treatment effect (LATE), i.e. effect among compliers (Angrist and Krueger, Reference Angrist and Krueger2001). In the present study's case, compliers are people prevented from tooth loss by water fluoridation. Water fluoridation is beneficial for all people but has a larger effect on people with a high risk of dental caries (Riley et al., Reference Riley, Lennon and Ellwood1999).
Most of the previous studies on the effect of tooth loss on depression were cross-sectional analyses. One exception is the study by Yamamoto et al. (Reference Yamamoto, Aida, Kondo, Fuchida, Tani, Saito and Sasaki2017), who followed older people in Japan for 3 years and found that being edentulous was a risk for developing depressive symptoms; however, some potential confounders, e.g. childhood environment, have not been adjusted for. Our approach, IV analysis, can consider all unmeasured confounders between tooth loss and depression. An IV approach can also mitigate the bias due to classical measurement error in the treatment variable (Angrist and Krueger, Reference Angrist and Krueger2001). As we relied on the government-reported information on water fluoride, respondents’ year of birth and county of residence when deriving the variation in the treatment variable, measurement error might have been mitigated compared to plain self-reported number of lost teeth.
Chronic stress and discomfort lead to hypothalamic-pituitary-adrenal (HPA) axis hyperactivity via dysfunction in the negative feedback system (Malhi and Mann, Reference Malhi and Mann2018). The accelerated HPA prolongs cortisol secretion, which is a major biological risk factor for depression (Malhi and Mann, Reference Malhi and Mann2018). Among the eight items of the PHQ-8, neurovegetative problems such as sleep problems, having little energy and less appetite/overeating were affected by tooth loss (eFig. 4 in the Supplementary Material). Another mechanism is explained by inflammation due to past or current periodontal diseases because inflammatory cytokines increase depressive symptoms (Malhi and Mann, Reference Malhi and Mann2018), although it is less likely in our estimate employing water fluoride as an instrument. Further, a decline in social interaction due to poor oral condition (Rouxel et al., Reference Rouxel, Heilmann, Demakakos, Aida, Tsakos and Watt2017) can also explain the result. People with fewer teeth might be less likely to communicate with others because of the problem of eating, speaking or smiling; and that might result in poor mental health outcomes.
The present study has limitations. We used three waves of repeated cross-sectional data, which is limited in terms of temporality between the treatment variable (tooth loss) and the outcome (depression); however, the instrument (exposure to drinking water fluoride in childhood) should be prior to losing teeth. We did not use survey weights although the proportion of gender in the BRFSS survey respondents was lopsided (Centers for Disease Control and Prevention, 2010); it is expected that the effect of tooth loss on depression will increase with the survey weights because the effect was larger in men than in women. It must be noted that we were not able to consider relocation, because only the current county of residence was available. We believe that this induces non-systematic misclassification because it is less likely that people select the county to live to receive the benefit of water fluoridation. In the USA, fluoridated toothpaste was rapidly utilised from the 1970s (Burt and Eklund, Reference Burt and Eklund2005). We considered the availability of fluoridated toothpaste by stratifying birth cohorts, and the results were similar. Many studies have shown that water fluoridation is still effective once other fluoride sources are available (Marinho et al., Reference Marinho, Higgins, Sheiham and Logan2004). We examined the correlation between county-level characteristics and the proportion of water fluoridation (eTable 1 in the Supplementary Material). Population size and income per capita showed moderate correlation (i.e. spearman's ρ >0.20), but adjusting for these variables did not change our findings. A further limitation is related to the self-reported outcome; however, the measurement has been well validated in clinical care settings (Kroenke et al., Reference Kroenke, Spitzer and Williams2001; Sekundo et al., Reference Sekundo, Stock, Jürges and Listl2019). Finally, the findings may not be directly transferrable to other countries or settings, although the prevalence of severe tooth loss in the USA is similar to the global population (Kassebaum et al., Reference Kassebaum, Bernabé, Dahiya, Bhandari, Murray and Marcenes2014).
In conclusion, we found sizeable causal effects of tooth loss on psychological well-being. This provides unique and novel evidence underpinning the urgency for taking better action to promote oral health. Further research to identify the causal effects of oral conditions on other aspects of population well-being is strongly encouraged.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S2045796021000287
Data
Data are available from the BRFSS website (https://www.cdc.gov/brfss/index.html).
Acknowledgements
We are grateful to BRFSS participants and appreciate Dr Matthew Neidell at Columbia University for providing the data of the Fluoride Census 1992 in a tidy format.
Author contributions
Y Matsuyama contributed to conception and design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; H Jürges contributed to data analysis and interpretation, critically revised the manuscript; M Dewey contributed to data interpretation, critically revised the manuscript; S Listl contributed to conception and design, data analysis and interpretation, critically revised the manuscript. All authors gave final approval and agreed to be accountable for all aspects of the work.
Financial support
This work was supported by the Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (JSPS KAKENHI Grant Number 19K19309). The funder of the study had no role in study design, data collection, data analysis, data interpretation or writing of the manuscript.
Conflict of interest
None.








