INTRODUCTION
In recent years the number of cases of Legionnaires' disease (LD) detected in Europe has risen sharply [Reference Joseph1]. This has been attributed to a range of factors including changes in diagnostic methods, improved surveillance systems and the identification of new sources of infection [Reference Joseph1, Reference Ricketts and Joseph2]. Most LD is community acquired. Sources widely reported to be linked to outbreaks include cooling towers [Reference Garbe3–Reference Nguyen5], spa pools and other aerosol-producing devices such as indoor fountains and food display misters [Reference Mahoney6–Reference Hlady8]. When outbreaks do occur they can be large, dramatic and result in considerable health-care costs [Reference Telford9, Reference Smith, Wild and Law10]. However, most cases that are identified are isolated sporadic cases where a source is never found. There is still little comparative information on the proportion of sporadic cases that acquire the disease from home, work, hospital or other environments. Because of this lack of information, and because every case could herald a large outbreak, public health authorities carefully investigate each new case of LD reported. The imperative is to identify any possible environmental source that could put others at risk. However, the costs and benefits of this approach have rarely been considered and there is currently no UK national guidance on what the threshold should be in triggering investigations of clusters of LD.
This paper is one of the first assessments of both the public health investigation costs and economic costs associated with investigating an apparent outbreak of LD. It also considers for debate whether the investigation threshold used is still valid when the incidence of isolated cases of LD continues to rise across Europe.
METHODS
An apparent outbreak was detected following the report of two cases of community-acquired LD to the South East London Health Protection Unit (SELHPU) on 17 July and 8 August 2005. A case definition for the outbreak was defined [using the standard Health Protection Agency (HPA) case definition] [11] as a person who had a clinical diagnosis of pneumonia with microbiological evidence of infection with Legionella pneumophila and a geographical association (having lived in or visited the South East London area during the 10 days before onset of illness) and became ill within the time period 10 July to 30 August 2005. Detailed investigations were performed in accordance with established national guidance on investigation and control of LD [12–14].
Epidemiological investigations
All patients and/or their close contacts were interviewed as soon as possible after a diagnosis was confirmed. We used standardized questionnaires to collect information on current illness, place of residence, possible risk factors and movements in the 2 weeks before illness onset. Face-to-face interviews were conducted in the hospital of treatment whenever possible with the aim of gaining a detailed case history. Further interviews were conducted with other close contacts or with the cases themselves using their personal diaries or calendars where necessary to clarify details of cases' movements. This included routes taken to and between home, workplaces, shops, pubs and other locations, including any trips out of South East London. The detailed travel histories enabled staff to map the movements of cases down to road and postcode level where possible. As each new confirmed case was identified, case histories were compared to look for possible epidemiological associations. Cases were re-interviewed if new information emerged from the investigation which required further checks linking them to a potential common source.
Clinical case ascertainment
In England and Wales there is no statutory requirement to report LD but microbiologists and doctors are requested to report confirmed or probable cases of LD to the local unit of the HPA. These are transmitted promptly to the National Surveillance Scheme for LD, coordinated by the HPA Centre for Infections (CFI). Active case searching was begun across South East London National Health Service (NHS) organizations. Letters about the increased incidence of LD cases locally were sent to all general practitioners, acute clinical and microbiology departments in the six acute NHS hospitals and NHS Direct services, alerting staff to the symptoms of the disease and reminding them to report any new cases immediately to SELHPU.
Other cases reported within the United Kingdom and across Europe were investigated for any contact with the South East London area through communication with local health protection units across England and Wales, and internationally through the European Surveillance Scheme for Travel Associated Legionnaires' Disease (EWGLINET) [15]. This was facilitated through the UK National Surveillance Scheme.
A diagnosis of L. pneumophila infection was established in the admitting hospitals using commercially available L. pneumophila urinary antigen kits. Laboratories were asked to submit all positive urine samples, together with any available respiratory samples from the respective patients, to the CFI. Urine samples were examined in the CFI using an in-house enzyme-linked immunoassay (EIA) specific for a subset (designated mAb2+ve) of L. pneumophila serogroup 1 strains [Reference Birtles16]. Isolation of L. pneumophila was attempted from respiratory samples using standard techniques [Reference Harrison and Taylor17] and where successful isolates were characterized by monoclonal antibody subgrouping [Reference Helbig18] and DNA sequence-based typing (SBT) [Reference Gaia19]. Culture-negative samples were further examined by PCR using an in-house L. pneumophila mip specific PCR with direct SBT being applied if they were positive [Reference Fry, Cianciotto, Abu Kwaik, Edelstein, Fields, Geary, Harrison, Joseph, Ratcliff, Stout and Swanson20].
Environmental investigations
Environmental investigations were performed by officers from five environmental health departments (Lewisham, Southwark, Bromley, Bexley and Wandsworth), the Health and Safety Executive (HSE) and staff from the CFI. Potential environmental sources were identified by reviewing the patients' movements in relation to the list of cooling towers registered with the local authorities, and local authority and HSE records of other known potential sources, e.g. car washes, train washes, fountains, spray cleaning devices, air scrubbers and irrigation equipment. In addition unregistered cooling towers and other potential sources were sought by the investigating officers walking the area and questioning the occupiers of commercial premises as appropriate. Letters were sent from local authorities to commercial premises reminding them of their legal obligations with reference to control and prevention of LD.
Samples were collected in containers containing sodium thiosulphate in sufficient quantity to neutralize any residual oxidizing biocide and sent for analysis at the HPA London Regional Food, Water and Environmental Services Laboratory (LFWEL) which is accredited by the United Kingdom Accreditation Service (UKAS) to ISO 17025 for the examination of water and environmental samples for the detection of Legionella. For each possible source any identified equipment or system was inspected visually, any maintenance and microbiological test records reviewed and microbiological samples collected. Domestic premises, when possible, were sampled according to HPA guidelines [Reference Lee and Surman21]. The microflora from the water samples (1 litre) were concentrated by a combination of filtration and centrifugation [Reference Gaia19] and used for the detection of Legionella by culture methods following ISO 11731 [22]. Internal quality controls were included with each batch of samples processed.
Estimating the health costs of the outbreak
Information of the costs involved in carrying out the investigation, control measures and management was obtained from the organizations involved once the outbreak was declared over. Each provided information on direct costs including staff resources dedicated to the investigation (both in time spent and actual payroll cost), travel costs, the costs of the domestic and environmental sampling (including laboratory costs, costs of transporting samples) and clinical microbiology costs. This did not include the costs of an organization's overheads nor other indirect costs incurred by the organizations. Estimates of treatment costs for all cases were derived from the information provided by the local Primary Care Trust, which is responsible for commissioning health care in the area where the outbreak occurred. These are estimates for the full costs of acute treatment including medical care, bed days, investigations and medicines. Other costs to patients themselves, their families or their employers were not collected in this exercise.
RESULTS
Epidemiology
Between July and October 2005 14 cases of community-acquired LD were reported associated with the South East London area. The epidemiological and microbiological investigations were consistent with a small initial cluster of six cases spread over about 6 weeks, followed by a second cluster with onset dates between 27 and 30 August 2005 (Fig.).
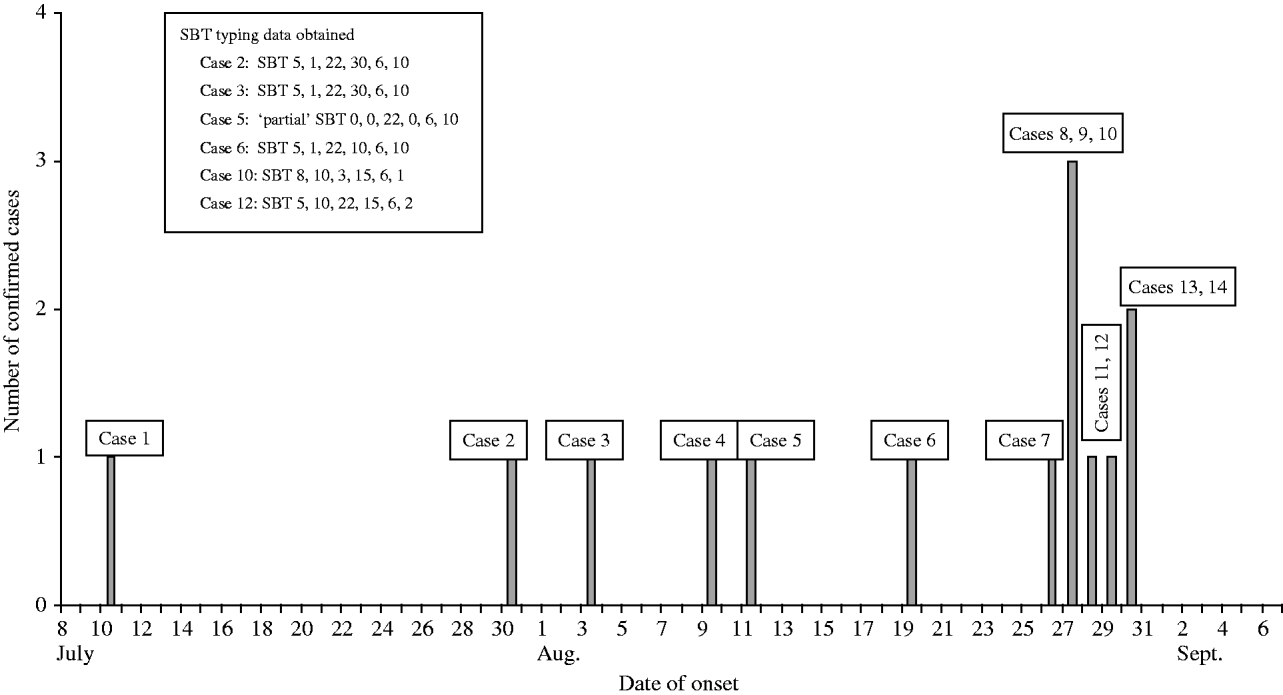
Fig. Confirmed cases of Legionnaires' disease in South East London, July–September 2005.
All cases were males aged from 31 to 73 years (mean 54 years). The backgrounds and lifestyles of the cases were, however, very different. Ten were actively employed and four retired. Twelve were residents of South East London and two worked in the area during the incubation period. Common areas of overlap could only be established for up to seven cases at any one time. Two cases had a history of travel abroad, but the timings strongly suggested that they acquired the disease locally.
The extensive investigation of case histories revealed no shared risk factors, although all but one were cigarette smokers. None of the cases had visited the location of a known potential LD source which was common to all other cases. The mapping permitted identification of areas where there had been overlaps in their movements. At a later stage in the investigation, staff at the HPA Centre for Emergency Preparedness and Response, Porton Down provided assistance with transferring the manually mapped case movements (and possible environmental source locations identified by the HSE and local environmental health departments) onto digital maps using Geographical Information Systems (GIS) technology. The GIS allowed a 500 m buffer zone to be plotted around case movements to highlight likely areas where common sources might be located by rating the cumulative risk of exposure for all cases on a scale from high to low.
Clinical
All cases had pneumonia confirmed by chest X-ray and positive urinary antigen tests. Twelve patients were admitted to three hospitals in South East London, although the hospital nearest the outbreak area received eight admissions. The other two cases were admitted to hospitals outside the area in Wales and Spain.
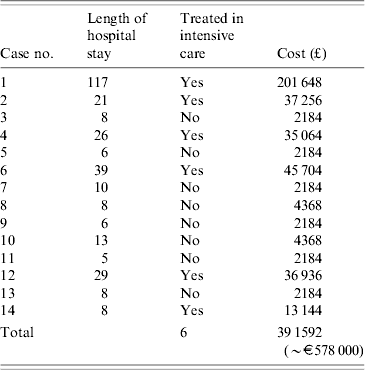
Duration of symptoms prior to admission varied from 2 to 8 days and included flu-like symptoms, fever, productive cough and shortness of breath. Seven patients presented with abdominal pain, vomiting and diarrhoea, and two patients developed confusion and slurred speech. Length of hospital stay ranged from 5 to 117 days (median 9 days) and six patients required treatment in intensive care. There were no deaths, but three patients suffered serious complications, one case remained in hospital for nearly 4 months and two required renal dialysis.
Clinical microbiology
Urine samples from 12 of the patients were submitted to the CFI. All were confirmed as positive for L. pneumophila serogroup 1 (mAb2+ve). Sputum samples were obtained from nine patients and L. pneumophila serogroup 1 was isolated from five of these: all were determined to be mAb subgroup ‘Allentown’. A further two patients (cases 5 and 7) were PCR positive for the L. pneumophila mip gene. Typing of the five isolates showed that two isolates were indistinguishable by phenotypic and genotypic methods (cases 2 and 3). Epidemiology confirmed that these cases had visited one defined location that had not been visited by any other case; however, no specific source was identified. Genotypic typing data from cases 6, 10 and 12 showed that they had been infected by three other strains. SBT was attempted directly from the two PCR-positive sputa but failed for case 7 and was only partially successful for case 5 yielding valid alleles for three of the six genes. This partial SBT profile indicated that case 5 was not infected with the same strain as cases 10 or 12 (Fig.). These data clearly showed that the 14 cases were caused by at least four distinct strains of L. pneumophila serogroup 1.
Environmental sampling and testing
During the investigation 176 environmental samples were collected including those from domestic premises, cooling towers, bus, car and train washes and fountains. These samples accounted for all the potential sources from commercial premises and from 11 of the 14 patients' homes. Access was not available to the remaining homes. The number of samples collected from these domestic premises varied from 1 to 10. None of the potential environmental sources or domestic premises was identified as positive for L. pneumophila. One implicated cooling tower could not be sampled for health and safety reasons as there was no safe access. This tower was closed on 12 August 2005 and was unlikely to be a common source. Precautionary control measures were also applied to some of the potential sources where applicable.
Direct economic costs
Investigation, control and management of the outbreak mainly involved staff from 11 organizations or independent units of organizations: six local authority environmental health departments, two local health protection units, the HSE, one local hospital microbiological laboratory, the HPA CFI and the Centre for Emergency Preparedness and Response, Porton Down. The response lasted over a period from the end of July to October. Total time spent on the investigation and control aspects of the outbreak across all organizations was about 1608 person hours, at an estimated minimum cost of £64 264 (€95 844) (Table 1). Most of the estimated staff time allocated to this outbreak was fairly equally divided between the epidemiological investigations (50%) and the environmental sampling and testing (45%); nevertheless the estimated total costs of the environmental investigations were somewhat higher than those for the epidemiological investigations – 48% compared with 39%.
Table 1. Estimated costs of the investigation, control and management of Legionnaires' disease outbreak in South East London (July–August 2005)

HPA, Health Protection Agency.
The estimated treatment cost incurred by the acute hospitals for the 14 patients was £391 592 (€584 000). Costs per patient ranged from £2184 to £201 648, with a mean cost of £27 971 (median £4368) (Table 2).
Table 2. Estimated treatment costs for all confirmed cases
DISCUSSION
Investigations in South East London over the summer of 2005 identified 14 cases of LD clustered in time and place. Whether they represented a single outbreak or several clusters and sporadic cases was discussed frequently during the incident. However, the investigation and management were undertaken as if the incident was an outbreak.
Microbiological typing subsequently showed that the cases were caused by at least four distinct strains of L. pneumophila serogroup 1, clearly excluding the possibility of a single point or continuing source outbreak. However the epidemiological and microbiological investigations were consistent with a small cluster of cases early in the investigation, followed by a second cluster with onset between 27 and 30 August 2005. Most of the first six cases appear to have been sporadic given the lack of distinct clustering in time and place and the microbiological finding of a number of distinguishable strains. The exceptions are cases 2 and 3, where the use of newly developed typing methods enabled us to confirm that these cases were caused by strains of L. pneumophila that were indistinguishable by phenotypic and genotypic methods. This was strong evidence, when taken together with a detailed history of common movements that these cases were caused by exposure to a common source, although none was identified. The later eight cases (nos. 7–14) are more indicative of a point source outbreak, given the tight clustering in time and place. The exception was case 12, which is most likely to be a sporadic case given the distinct epidemiology and typing data. Unfortunately, most of these later cases were unable to produce sputum samples. Consequently there was a lack of microbiological evidence to confirm a common Legionella strain. Despite the lack of clinical samples in these later cases it should be noted that overall a substantial proportion of cases were culture proven and this enabled typing to be undertaken to refine the epidemiology. This success was due largely to the good working relationships between staff in the organizations involved which led to the prompt referral of respiratory samples from urinary antigen-positive cases: in our experience isolation rates exceed 50% where such samples are available (T. Harrison, personal communication).
It is recognized that the active case finding undertaken in this investigation may have increased ascertainment in the area and identified cases that might otherwise have remained undetected. However, the 14 cases of community-acquired disease in South East London in 2005 compares with only two cases reported from this area in 2004 [Reference Joseph23].
Changes in clinical practice and diagnostic testing (e.g. greater use of the urinary antigen detection test in hospitals) may also account for some of the overall rise in cases. A real increase in incidence may also have occurred, as other European countries also reported a greater than expected seasonal incidence of community-acquired cases around the same time [Reference Ricketts and Joseph2]. Since the large outbreak in North West England in 2002, the annual number of reported cases in England and Wales rose from around 220 to over 300 per year. About 25% of these cases were associated with known clusters or outbreaks and almost 50% of them associated with travel, either in the United Kingdom or abroad [24]. Case-fatality rates have remained stable over the past few years at 10–13% the exception being for healthcare-associated cases where the fatality rate has been between 40% and 50% (source http://www.hpa.org.uk/infections/default.htm). These data do not indicate that increased ascertainment is solely accountable for the rise in reports, as fatality rates would be expected to decline if a greater number of less severely ill cases were being detected.
The fact that no common source for some or many of the cases was found does not necessarily mean that one did not exist. National data suggest that a common source was identified in only around 50% of clusters/outbreaks that occurred in England and Wales since 1980 (C. Joseph, personal communication). The prompt and exhaustive environmental sampling undertaken is likely to have ensured that all potential sources were identified, sampled and controlled. However, the rapid action taken by local authorities and the publicity around the outbreak may have prompted local businesses in the area to take precautionary or remedial action to ensure they were in compliance with the UK codes of practice [25]. This may have not only prevented further cases, but also prevented isolation of legionellae from any of the subsequent environmental water samples. In any investigation there is always the conflict between taking samples quickly before any remedial dosing takes place, and protecting the public health from an ongoing source by precautionary disinfection. When an investigation is prolonged, it is inevitable that some potential sources may only be identified after remedial action has taken place.
Confidence that the laboratory results were not false negatives in this outbreak can be assured from the rigorous internal controls. In addition, during the outbreak period legionellae were isolated from 131/767 samples (17%) from a range of sites and areas within the London and the Home Counties not associated with the outbreak. The percentage of positive samples (17%) was the same between August and October 2006 (J. Lee, personal communication).
GIS was used to map all case movements, potential sources and overlay a 500 m buffer zone around likely areas of overlap. The use of GIS mapping occurred rather late in the investigation and in this situation it did not add much to the progress or outcomes as existing data had already been mapped manually. GIS could prove a useful tool in a bigger investigation or if used from an earlier stage. Once electronically mapped it is easier to update case and source information and potential overlaps.
Costs of investigating clusters of LD
The estimated minimum cost of investigating and managing this outbreak (including epidemiological, clinical, microbiological and environmental sampling) was £64 264 (€95 844). Considerable staff time (1608 h) was provided at short notice by the six local authority environmental health departments, the local South East London HPU, and staff from the HPA CFI Colindale. It should be recognized that this is an underestimate of the true costs to the organizations involved. Only the cost of actual person-time committed to the investigation was estimated, which did not include overhead or indirect costs. In addition, there were opportunity costs for all the organizations involved. Undertaking work for the outbreak was at the expense of other work that had to be delayed or covered by other people.
Nevertheless, estimated costs of the outbreak investigation were overshadowed by the enormous costs of treating the 14 cases of LD at the hospitals of admission. Of the overall costs of £455 856, only 14% was spent on investigation and control of the outbreak compared with 86% on patient treatment. The estimated treatment costs ranged from £2148 to £201 648 per patient (Table 2), with the mean treatment cost being £27 971. The total treatment costs were at least £391 592 (€584 000) and may have been higher as only direct patient treatment costs were estimated. Costs associated with enhanced case finding, i.e. chest X-rays or urinary antigen testing in all patients attending A&E with pneumonia-like symptoms were not included. Eight cases were in-patients in the same hospital, placing a disproportionately high burden in terms of service pressure and costs on one service provider. It was fortunate that the patients who were particularly ill were admitted to several different hospitals because demand for beds (especially intensive care), staff and other resources did not result in further hospital ‘costs’ being incurred (e.g. cancelling elective surgery or filling intensive care facilities) as has happened in other larger outbreaks of LD when most patients were admitted to a single hospital [Reference Smith, Wild and Law10].
There has been no follow-up study to estimate the indirect social, health and economic costs to the patients, their families and employers of the acute illness or its long-term sequelae. It is probable that these would be significant. One follow-up study of the health of 122 survivors of an outbreak of LD in The Netherlands showed that most reported impacts on their health-related quality of life and 15% had symptoms of post-traumatic stress disorder which persisted for at least 1·5 years [Reference Lettinga26].
Costs of LD outbreak management have not been published in detail before, although reference has been made to the estimated extra hospital costs of £5 million for diagnosis and treatment in a large UK outbreak in 2002 [Reference Telford9]. Further studies of the economic costs of LD outbreak investigations are needed. These should include longer term follow-up of cases to estimate the wider indirect costs to patients, their families and health-care systems [Reference Lettinga26]. With such information it might be possible to create a cost model that could be applied more widely to other outbreaks and sporadic cases.
Conclusion: a threshold for investigation?
Should we treat every new case as if it is the start of an outbreak or should we have an agreed threshold for investigation of clusters of LD? It could be argued that the continuing increase in incidence of LD across Europe justifies investigation of every case to determine whether cases are simply clustered in time or linked to specific outbreaks. There is no UK national guidance on what the threshold should be for triggering investigations of clusters of LD. In England, as a minimum standard every case should be subjected to a 2-week exposure history and a check of major risk factors. When cases are clustered in space or time this warrants further investigation to exclude a common source. The public health response to two or more linked cases should ideally be managed within the context of what is known locally about the geographical and industrial landscape and population density. There is currently no ‘right’ answer to this issue. For example two community-acquired cases linked in time and place in a rural area would be rare and should generate intensive investigations, whereas two cases linked in time and place in a large town or city are fairly common and may be due to different sources that may never be identified because of the multiple opportunities for infection in this setting.
As the number of community-acquired cases seems to be increasing each year, particularly in urban settings, this level of investigation will have serious implications for the workload of health protection agencies. The cost-effectiveness of investigating all clusters is likely to diminish in the long run. Resources may be better used in ensuring effective control measures are rigorously applied to potential sources of infection and that managers of these systems are fully aware of their legal responsibilities for maintaining Legionella risk-free systems. These measures could be adopted in conjunction with good public health practice such as the regular use of media publicity to inform the local population about LD and its aetiology.
Ascertainment of cases is rising each year, partly driven by more testing for Legionella infection in patients with pneumonia, greater use of the urinary antigen detection method and more awareness of the disease in the general population. In the absence of a known or suspected point source outbreak, incidence rates per million population per region could be used to provide baseline incidence levels, beyond which health protection units should consider a trigger for more extensive investigations.
Improved knowledge of the cost-effectiveness of such investigations may better inform development of future guidelines for the investigation and management of LD against this background of rising incidence and health-care costs. Whether the threshold for investigation should be developed in relation to observed incidence rates, to the cost-effectiveness of investigations, or both should be debated further.
ACKNOWLEDGEMENTS
We thank the following for contributing to the investigation and management of the outbreak between July and October 2005: Diana McInnes, South East London Health Protection Unit; Barry Walsh, South West London Health Protection Unit; Deborah Turbitt, North East and Central London Health Protection Unit; Katherine Lewis, HPA London; Helen Maguire, HPA London; David Avenell, Leslie Jones, Centre for Emergency Preparedness and Response, HPA Porton Down; Tay Potier, Graham Willes, London Borough of Lewisham Environmental Health Department; Clive Cain, London Borough of Bexley Environmental Health Department; Beryl Morgan, Sailesh Chudasama, London Borough of Southwark Environmental Health Department; Paul Lehane, London Borough of Bromley Environmental Health Department; Lorraine Charles, Emma Stiles, Health and Safety Executive, London; John Kensit, Queen Mary's Hospital, Sidcup; Jim Wade, Kings College Hospital.
DECLARATION OF INTEREST
None.







