Introduction
Currently, tuberculosis (TB) remains a serious public health threat. According to WHO (2019), an estimated 10.0 million people fell ill with TB, and an estimated 1.4 million TB deaths occurred worldwide in 2018 [1]. As known, all factors associated with immune status may have an effect on the development of TB. Until now, several factors, such as HIV infection, undernutrition, diabetes, smoking, alcohol consumption, transplant recipients and malignancy [Reference Zachariah2–Reference Torre-Cisneros8], have been identified in the development of pulmonary tuberculosis (PTB). Similarly, immune-compromised status is also a major factor preceding aspergillosis, and the above-mentioned risk factors are also shared in the development of invasive aspergillosis (IA), such as HIV infection [Reference Wallace9], transplant recipients [Reference Sole10], malignancy [Reference Yan11] and smoking [Reference Ali12], which has been investigated in various studies. On the other hand, pulmonary cavity usually is a result of a previous TB, and these residual cavities become infected with Aspergillus and pulmonary aspergillosis then develops following inhalation of airborne fungal spores [Reference Page13]. This suggests that retreatment TB cases are at a high risk of developing IA.
IA is a common infection in immune-compromised patients. Despite advances in therapy, IA remains a serious and fatal opportunistic infection. The prevalence of aspergillosis complicating TB has been reported in several studies from different countries, and the results demonstrated that the prevalence varied widely, and was up to 25% in active TB cases [Reference Hedayati14–Reference Chu18]. Additionally, in a recent meta-analysis, a pooled rate of Aspergillus coinfection among patients with pulmonary TB was reported at 15.4% (95% CI 11.4–20.5) in Asia and Africa [Reference Hosseini19]. This variance may be explained by the difference in the diagnostic criteria and geographical distribution of IA. However, in China, the prevalence of IA in patients with suspected PTB remains unclear. Hence, in this retrospective study, we aimed to evaluate the prevalence of IA in suspected PTB.
Materials and methods
The study protocol was approved by the ethical committee of the Shandong Provincial Chest Hospital (SPCH). Written informed consent was waived by the ethical committee of SPCH because of the retrospective study design and an absence of personal information. The investigations were carried out in accordance with the Declaration of Helsinki.
During a period of 1 year (from January 2016 to December 2016), consecutive patients with suspected PTB were included in SPCH. Data, including demographic information and underlying diseases, were collected from medical records. PTB were all confirmed by mycobacterial culture (Lowenstein–Jensen medium). Non-tuberculous mycobacterial (NTM) disease was diagnosed according to American Thoracic Society criteria [Reference Griffith20]. Retreatment TB was defined as a new TB diagnosis in a patient who had previously completed TB treatment.
In suspected IA cases, bronchoscopic alveolar lavage fluid (BALF) and serum samples were collected and sent for galactomannan (GM) assay (DNK Ltd, Tianjin, China). Computed tomography-guided percutaneous transthoracic needle biopsies were performed in suspected IA cases and then processed for pathological examination. BALF, sputum and tissues were processed for fungus culture. Two samples were cultured on two Sabouraud agar plates separately and incubated at 37 and 45°C for up to 5 days. The isolated fungus was identified by its colony characteristics and morphological features.
IA were diagnosed as proven or probable according to the criteria of the 2008 EORTC/MSG definitions [Reference De Pauw21]. Briefly, proven IA was defined by histology showing hyphal tissue invasion (Gomori methenamine-silver, with hyphal walls staining dark) and microbiological proof of Aspergillus infection. Probable IA was established according to radiologic findings plus a positive BALF culture, or a positive GM assay (ELISA method) on BALF (⩾0.90 μg/l) or serum (⩾0.75 μg/l).
All analyses were performed using SPSS version 16.0 (SPSS, Chicago, IL, USA). Due to small numbers of IA patients, a descriptive analysis was performed. The continuous data are presented as the mean ± s.d. and categorical data as frequencies (percentage). The prevalence was estimated and the corresponding 95% confidence interval (CI) was also calculated.
Results
During the study year, 9125 sputum collected from 7141 suspected PTB patients were sent for mycobacterial culture (Fig. 1). Of them, 1507 patients had a positive mycobacterial culture, with a mean age of 45.6 (s.d. 19.9) years old and a female:male ratio of 1:4 (female, n = 487, 41.2 (s.d. 16.7) years; male, n = 47.7 (s.d. 19.7) years). Subsequently, NTM diseases were diagnosed in 82 (5.4%) patients, and TB in 1315 (87.3%) patients, the remaining 110 (7.3%) patients were excluded for the final diagnosis of NTM diseases could not be established. Of the 1315 PTB patients, 232 (17.6%) were retreatment cases. HIV status was examined in 1499 suspected PTB patients (mycobacterial culture, +), and all of them were HIV-negative.
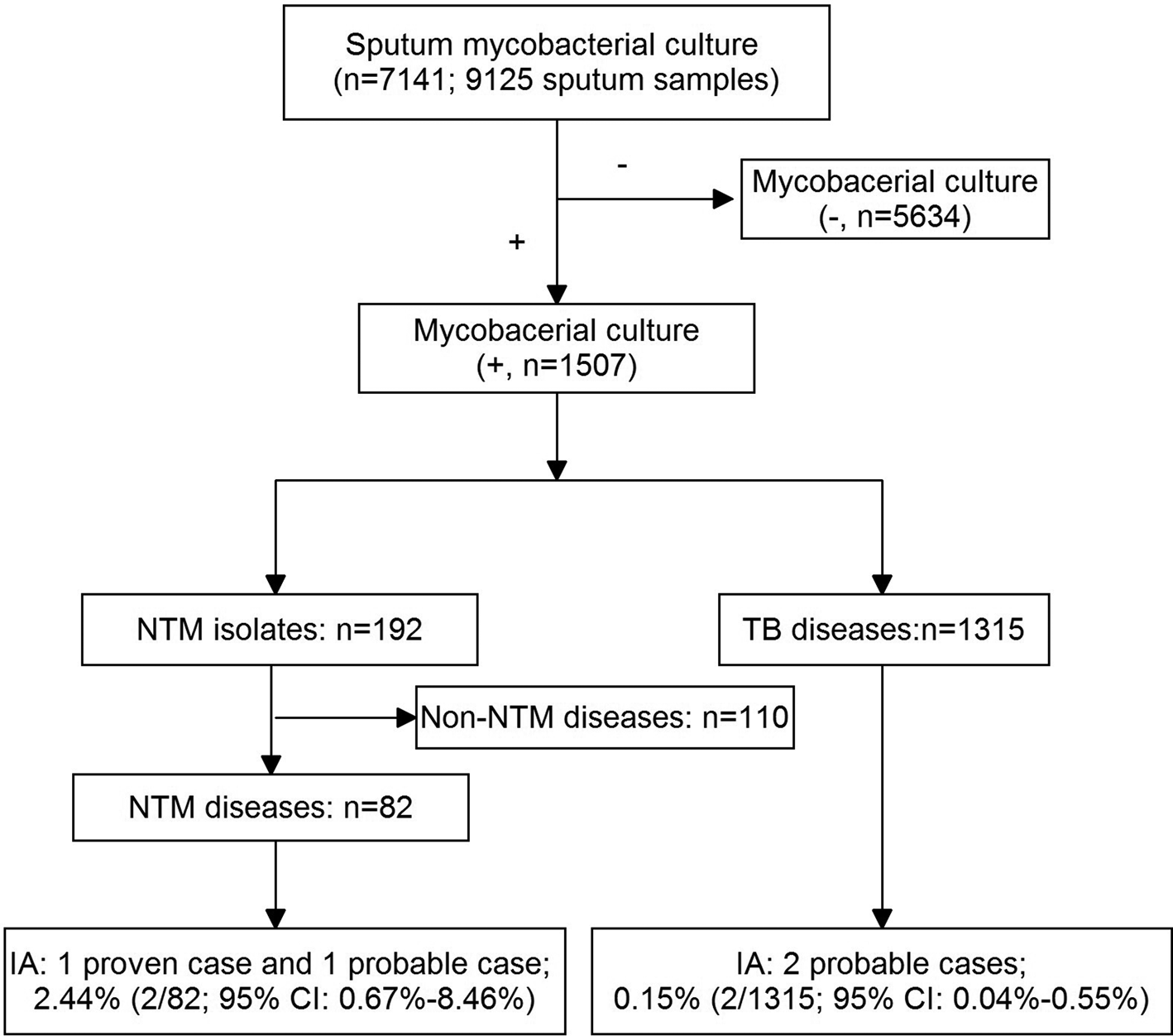
Fig. 1. A flow chart of the patients included in the study.
A total of four IA patients were identified in this study, including one proven case and three probable cases. The proven case was confirmed using tissue culture, probable cases were established according to radiologic findings plus a positive BAL culture in one patient, and a positive GM on BAL in two patients. The four IA patients all exhibited cavities in both lobes on radiograph. Additionally, all IA patients were administrated with voriconazole orally (200 mg twice a day) for 6 months.
Among the patients with NTM disease, two patients were diagnosed as IA (one proven and one probable). Therefore, a prevalence of 2.44% (95% CI 0.67–8.46%) of IA in NTM diseases was estimated. Likewise, two probable IA patients were diagnosed in culture-confirmed PTB patients, and a prevalence of 0.15% (95% CI 0.04–0.55%) of IA in PTB was then estimated. Interestingly, the two IA complicating TB patients were all retreatment cases. Therefore, among retreatment TB cases, the prevalence of IA was 0.86% (95% CI 0.24–3.09%).
Discussion
To our knowledge, this is the first report to systematically evaluate the prevalence of IA in active PTB in China. In the study, a low prevalence was observed, and these cases were all associated with common risk factors, such as NTM disease or retreatment TB.
Interestingly, raised Aspergillus-specific antibodies were observed in TB patients when administered with anti-TB therapy [Reference Kwizera22]. For example, in a study by Chu et al. [Reference Griffith20], a serological assay was used to evaluate the prevalence of Aspergillus infection, with a prevalence of up to 25% in patients with old TB or bronchiectasis. Although several different methods for antibody against Aspergillus were used to define the infection and have different sensitivities [Reference Iwata, Miwa and Takagi23], this phenomenon suggests that Aspergillus infection was common to complicate active PTB. Additionally, a high prevalence of Aspergillus infection was estimated at 12–22% in African TB patients with cavities [Reference Parkes-Ratanshi24]. In general, these data mentioned above mean a possible association between IA and TB. As known, the diagnostic criteria for the Aspergillus infection are arbitrary and based on expert opinion [Reference Denning25], and therefore varied between studies. Hence, standardised and validated tests, such as tissue culture and pathological examinations, are recommended.
However, the prevalence of IA was low in active TB cases. For example, in a study from Iran, IA was observed as co-infection with Mycobacterium tuberculosis in 3.72% of suspected PTB cases [Reference Jabbari Amiri16]. Oladele RO et al. found 8.7% of patients with smear-negative TB and treatment failure can establish an alternative diagnosis of chronic pulmonary aspergillosis (CPA) based on serological assay, chest X-ray, culture and symptoms [Reference Oladele26]. In addition, a recent report from China showed that IA complicating active TB occurs in a minority (about 5%) of paediatric patients with chronic granulomatous disease [Reference Gao27]. In our study, a lower prevalence of IA in active PTB patients was observed. Geographical distribution may be responsible for the difference in the prevalence of IA between different studies [Reference Hosseini19]. As reported, the prevalence of Aspergillus infection showed a considerable geographical variation, and China had an intermediate prevalence of CPA in previous TB patients [Reference Peman and Salavert28, Reference Smith and Denning29].
IA usually complicates several underlying lung diseases, such as previous PTB, NTM infection, COPD and bronchiectasis [Reference Smith and Denning29]. The existing data have shown that a previous PTB is associated with the occurrence of CPA [Reference Page13]. Furthermore, a high prevalence of Aspergillus sensitisation was reported in PTB-related fibrocavitary disease [Reference Dhooria30]. In high TB burden countries, TB is usually the most common primary underlying condition in the development of CPA [Reference Aguilar-Company31]. However, compared with other lung conditions, it has a relatively good prognosis. Our findings are consistent with these features. Four IA patients were diagnosed, two of them were retreatment TB cases, another two of them had NTM disease, and all of them exhibited cavities on radiograph. As reported, the Aspergillus infection was frequently seen in those with chest radiography cavitation than without it [Reference Page13]. This suggests that PTB patients should have chest radiography and that those with cavities should be monitored for Aspergillus to facilitate early treatment.
This study has several limitations. First, due to the economic and geographical condition of the area (Shandong) in China, our data may be further used to assess the situation of other areas in eastern China. However, when generalised to other areas, such as most areas in western China, our data may have limited clinical value. Second, due to the low incidence of the invasive forms of Aspergillus infection, a small number of patients included implies low statistical power to estimate the prevalence. Third, this is an institutional study, so the results may have a selection bias. Fourth, as known, most clinical and radiological characteristics of PTB overlapped with IA. Therefore, although all TB cases were culture-confirmed, some patients complicating IA may be diagnosed as having only TB.
Conclusions
Our data demonstrated that the prevalence of IA is low in active PTB patients. However, when high-risk factors, such as cavities on chest radiograph and some specific underlying diseases, are encountered in a suspected TB patient, we emphasise that further investigations are required, and empirically treatment for IA might be warranted.
Financial support
This study was supported by the Science Research and Technology Development Plan of Baise City (20201705).
Conflict of interest
None.
Data availability statements
Data are available upon request from the corresponding authors (WMS and HH).






