INTRODUCTION
Listeria monocytogenes is a rare, but important cause of human illness. Infection is characterized by febrile gastroenteritis, sepsis, and meningitis and may result in death or fetal loss. Nearly all cases of human listeriosis are foodborne [Reference Schuchat1–Reference Hof5]. Those considered at risk for severe illness include the elderly, pregnant women and newborns, and immunocompromised persons. In 2003, FoodNet, which conducts active, population-based surveillance for laboratory-confirmed cases of selected enteric pathogens, estimated that the incidence of listeriosis in pregnant women was 2·7 cases per million, with a higher incidence in Hispanic women than non-Hispanic women [Reference Voetsch6].
Listeriosis during pregnancy may result in fetal loss, premature labour, neonatal infection, and neonatal death. In the USA, an estimated 16% of reported listeriosis cases occur in pregnant women [Reference Voetsch6]. Listeria infection is most likely to develop during the third trimester as well as in women with multiple gestations [Reference Doganay7, Reference Mascola, Ewert and Eller8]. However, products of conception are not routinely cultured so it is unclear whether listeriosis is a common cause of early fetal loss.
Most listeriosis cases that occur during pregnancy are in healthy women without additional predisposing factors [Reference Mylonakis9, Reference Seigman-Igra10]. While maternal illness is mild, and often asymptomatic or self-limited, fetal and neonatal infection can be severe; perinatal case-fatality proportions range from 22% to 45% [Reference Mylonakis9–Reference Schuchat14]. A case series of pregnancy-associated listeriosis showed that 20% of cases resulted in spontaneous abortion or stillbirth; of the remaining pregnancies, 68% resulted in neonatal infection [Reference Mylonakis9].
Dietary risk factors for infection include ready-to-eat foods such as soft cheeses, hot dogs, delicatessen meats, melons eaten at a commercial establishment, and hummus prepared at a commercial establishment [Reference Schuchat1, Reference Schwartz2, Reference Varma15–Reference Gottlieb17]. Several outbreaks of listeriosis in the USA, primarily in pregnant Hispanic women, have been associated with Mexican-style soft cheeses made from unpasteurized milk [Reference MacDonald18, Reference Linnan19]. When compared to case-patients with sporadic non-perinatal listeriosis, pregnancy-associated case-patients were more likely to have consumed Mexican-style cheese, ice cream, or yoghurt [Reference Mascola20].
Much of what has been described about pregnant women with listeriosis has been reported from other countries, as a result of outbreaks, and from FoodNet sites. Food exposures specific to pregnant women with Listeria infection in the USA have been described only through surveillance of a single county in Los Angeles, California [Reference Mascola20]. Data from the Listeria Initiative expands available information and allows for analysis of food exposures in pregnant women with Listeria infection throughout the USA. To describe illnesses and food exposures associated with pregnancy-associated listeriosis, we analysed surveillance data from the Listeria Initiative from 2004 to 2007. This analysis is the first one since the inception of the Listeria Initiative in 2004.
METHODS
Data sources
The Listeria Initiative was launched in 2004 as a tool to aid investigations of listeriosis clusters by using a standardized, extended case-form questionnaire to obtain food exposures in all human cases of listeriosis. Patients are interviewed as they are recognized, allowing for better recall and more accurate food consumption histories.
Listeriosis cases are reported voluntarily to CDC on a standard reporting form completed by personnel at state and local health departments. The case report includes information on patient demographics (e.g. age, sex, race/ethnicity), clinical information (e.g. symptoms, type of illness, patient outcomes), laboratory information (e.g. date of collection and source of the specimen that yielded the Listeria isolate), and epidemiological information (e.g. a detailed history of food exposures). As soon as cases are identified, patients are interviewed about foods that are considered high risk for Listeria contamination that were consumed during the 4 weeks before the illness began. Food items listed in the questionnaire include pre-packaged and delicatessen luncheon meat, soft cheeses, ready-to-eat delicatessen-style salads, seafood, fruit, milk, and other dairy products. If the date of onset was not available, the food history was obtained for the 4 weeks before the specimen collection date. For pregnancy-associated illnesses in which the mother was asymptomatic, food history is collected for the 4 weeks prior to giving birth. The information is stored in a single database. States are requested to complete the case report form for all patients with positive Listeria cultures.
Case definitions
For surveillance purposes, a case was defined as the isolation of L. monocytogenes from a clinical sample of a normally sterile site. Cases were classified as pregnancy-associated if illness occurred in a pregnant woman or in an infant aged ⩽28 days. Each mother–infant pair was counted as a single case. When the history of exposures was recorded, the mother was considered to be the case-patient, even when only the infant was clinically ill. Invasive isolates refer to isolates obtained from a normally sterile site, such as blood or cerebral spinal fluid (CSF). The term neonate refers to any live-born infant aged ⩽28 days and fetal loss describes situations in which the pregnancy resulted in miscarriage or stillbirth. For ill neonates, illness was characterized as early onset if the date of onset occurred within the first 7 days of life. Neonates with onset of listeriosis 8–28 days after birth were classified as late onset. In cases where date of onset was not obtained, the date of the first positive Listeria culture was used. Admission and discharge dates were used to determine the length of hospitalization.
Data on national number of births by ethnicity of the mother, rates of multiple births, and perinatal fatality rate were obtained from the National Center for Health Statistics and are based on all birth and death certificates filed in all 50 U.S. states and the District of Columbia during 2005 [Reference Martin21, Reference MacDorman and Kirmeyer22]. The percentage of multiple births was calculated using the same procedure used by the National Center for Health Statistics and is the number of individual live infants resulting from multiple gestations divided by the number of live births. The perinatal fatality rate was defined as the number of fetal and neonatal deaths occurring from 20 weeks of gestation to 28 days after birth divided by the total number of live-born infants and fetal losses. We examined pregnancy-associated cases of listeriosis that occurred during 2004–2007. Data were analysed using SAS software, version 9.1 (SAS Institute Inc., USA).
RESULTS
Study population
A total of 758 listeriosis cases were reported to the Listeria Initiative during 2004–2007, of which 128 (16·9%) were considered pregnancy-associated. Cases were reported from 30 states, with pregnancy-associated cases originating from 20 states. The median age of mothers was 28 years (range 15–45 years); the majority (n=81) were white (80·2%), followed by 15 African Americans (14·8%) and five Asians (5·0%). Ethnicity was reported for 106 (82·8%) pregnancy-associated case-patients. Of the pregnancy-associated case-patients, 56 (52·8%) described themselves as Hispanic compared to 985 505 (25·6%) of mothers giving live birth during 2005 [odds ratio (OR) 3·25, 95% confidence interval (CI) 2·2–4·8]. Of pregnancy-associated cases, Hispanics accounted for 3/6 (50·0%) patients who were still pregnant at the time of interview, 12/21 (57·1%) patients with fetal loss, and 41/79 (51·9%) of live births. Most interviews (67·7%) were conducted with the case-patient (mother). Parents and spouses were the most common surrogates for cases when the case-patient could not be interviewed.
Illness and outcomes – mothers
Listeria was isolated from the neonate's blood (34·9%), the mother's blood (33·3%), the neonate's CSF (21·4%), the placenta (19·8%), amniotic fluid (4·8%), or some other site (10·3%). Some of the mother–infant pairs (19·0%) had multiple sites of isolation. Of 110 mothers, 31 (28·2%) were reported as having no illness; 22 (20·0%) were septic; 20 (18·2%) had a non-specific flu-like illness; 25 (22·3%) had other signs and symptoms, such as preterm delivery, abdominal pain, or decreased fetal movement; nine (8·2%) had febrile gastroenteritis; and eight (7·3%) were reported as having amnionitis. Symptoms associated with the mothers' illnesses most commonly included fever (73·4%), chills (51·7%), headache (46·1%) and muscle aches (37·4%). Other symptoms reported were vomiting (25·6%), stiff neck (22·0%), and diarrhoea (⩾3 loose stools/day) (19·3%). Nearly half (47·8%) of the pregnant women were hospitalized for Listeria-related illness. The length of hospitalization ranged from 0 to 14 days with a median stay of four nights. There were no maternal fatalities. Seven patients (5·5%) were still pregnant at the time of interview.
A large proportion of pregnancies resulted in fetal loss (26/128 cases, 20·3%), including one set of twins, bringing the number of fetal losses to 27. Week of gestation was recorded for 25 (93%) of the fetal losses; 12 occurred during or after week 20. Fetal loss occurred at a median of 19 weeks (range 8–30), with 80% of the losses occurring between 15 and 24 weeks of gestation. Pregnancies were more likely to be associated with fetal loss or infant death if Listeria was isolated from the mother's blood (25/42, 59·5%), in which nearly three-fifths resulted in fetal or infant death compared to isolates from sources other than blood (13/73, 17·8%) (OR 8·26, 95% CI 3·52–19·38). Pregnancies where the mother was either not cultured or tested negative for Listeria still accounted for a large proportion of pregnancies resulting in fetal or infant losses (34·2%).
Illness and outcomes – neonatal disease
Most of the pregnancies, 95/128 (74·2%), resulted in live births. Live births occurred between 25 and 42 weeks' gestation with a median gestation period of 36 weeks. Five mothers gave birth to twins, bringing the number of live infants to 100. Multiple births accounted for 10·0% of live infants reported to the Listeria Initiative compared to 3·4% of all births nationwide in 2005 (OR 3·18, 95% CI 1·65–6·11). Clinical manifestations in newborns included bacteraemia (36·5%), meningitis (32·9%), pneumonia (5·9%), other (15·3%), and unknown (14·1%). Examples of other illnesses included gastroenteritis and fever. No illness was reported in 7% of newborns. Most of the ill neonates, 47/76 (61·8%), had early illness onset (within the first 7 days of life). Of the neonates, 76/84 (90·5%) were hospitalized and 4/71 (5·6%) later died. Overall, the perinatal fatality rate was 14·3%, 13 times higher than the perinatal mortality rate reported in the USA during 2005 (1·1%) [Reference MacDorman and Kirmeyer22]. For newborns, the median length of hospitalization was 14 nights (range 4–50).
Food exposures
The most commonly reported food exposures in pregnancy-associated case-patients were ice cream (54·6%), butter (53·8%), yoghurt (52·2%), hot dogs (51·5%), and delicatessen ham (50·0%). Other exposures included cantaloupe (39·3%), chicken delicatessen meat (16·8%), turkey breast delicatessen meat (34·0%) and Mexican-style cheese such as queso fresco (27·4%). Two of the pregnancy-associated case-patients drank raw (unpasteurized) milk.
Pregnancy-associated case-patients were more likely to have eaten Mexican-style cheese such as queso fresco (OR 2·56, 95% CI 1·55–4·24), whole milk (OR 2·38, 95% CI 1·51–3·75), raw (unpasteurized) cheese (OR 2·51, 95% CI 1·09–5·75), yoghurt (OR 1·82, 95% CI 1·16–2·87), or hot dogs (OR 1·55, 95% CI 1·01–2·37) during the month before onset than were non-pregnancy-associated case-patients. A minority (12·2%) of pregnancy-associated case-patients who consumed hot dogs reported that they did not heat their hot dogs compared to 2·4% of non-pregnancy-associated case-patients (OR 5·59, 95% CI 1·43–21·86). Pregnancy-associated case-patients were less likely than non-pregnancy-associated case-patients to report consumption of coleslaw (OR 0·57, 95% CI 0·35–0·94), pastrami or corned beef (OR 0·20, 95% CI 0·06–0·64), honeydew melon (OR 0·56, 95% CI 0·31–0·99), and other milk (such as soy, chocolate, buttermilk) (OR 0·51, 95% CI 0·26–0·97) during the month before onset of illness.
Comparing Hispanic and non-Hispanic pregnancy-associated case-patients, non-Hispanic patients were more likely to report consumption of turkey breast delicatessen meat (OR 6·02, 95% CI 2·10–17·25), pasta salad (OR 5·67, 95% CI 1·16–27·62), butter; (OR 5·13, 95% CI 1·91–13·80), hot dogs (OR 3·74, 95% CI 1·50–9·33), and bologna (OR 2·86, 95% CI 1·04–7·89), while Hispanic patients (21/43, 48·8%) reported Mexican-style cheese consumption more often than non-Hispanic patients (5/43, 11·6%) (OR 7·25, 95% CI 2·40–21·96) (Table 1). Hispanic and non-Hispanic pregnancy-associated case-patients reported similar raw (unpasteurized) cheese consumption [5/38 (13·2%) vs. 4/42 (9·5%), respectively]. However, in non-pregnancy-associated case-patients, consumption of raw (unpasteurized) cheese (OR 5·70, 95% CI 1·84–17·64) was associated with Hispanic ethnicity.
Table 1. Bivariate analysis of dietary risk factors for Listeria monocytogenes in non-Hispanic and Hispanic pregnancy-associated cases reported to the Listeria Initiative, USA, 2004–2007
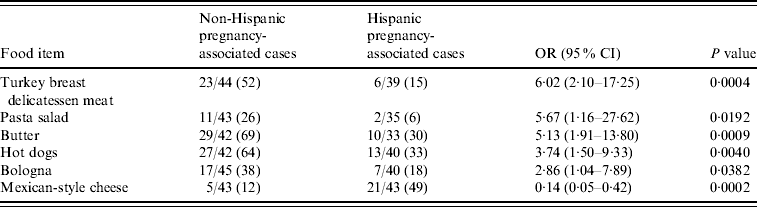
OR, Odds ratio; CI, confidence interval.
DISCUSSION
Our analysis demonstrates that an important proportion of reported listeriosis cases (16·9%) are comprised of pregnant women. Common exposures included foods previously recognized to be associated with Listeria infection [Reference Schwartz2, Reference Varma15–Reference Linnan19, Reference Olsen23, Reference Boggs24]. Pregnancy-associated case-patients were more likely than non-pregnancy-associated case-patients to report consumption of hot dogs, Mexican-style cheese, whole milk, yoghurt, or raw cheese. The finding that pregnancy-associated case-patients were more likely to consume Mexican-style cheese or yoghurt than non-pregnancy-associated case-patients has been previously reported [Reference Mascola20].
When compared to mothers giving live birth during 2005, pregnancy-associated case-patients were more than three times more likely to report Hispanic ethnicity, indicating that continued educational campaigns for pregnant Hispanic women are needed. During 1996–2002, the incidence of pregnancy-associated listeriosis in Hispanic women was higher than the incidence in of non-Hispanic women, although the rate in pregnant Hispanic women fell between 1998 and 2003 [Reference Voetsch6]. Hispanic pregnancy-associated case-patients reported significantly higher rates of Mexican-style cheese consumption than non-Hispanic pregnancy-associated case-patients. This suggests that ethnic variation in dietary patterns may explain part of the disparity seen in pregnant Hispanic case-patients.
Cheese produced with raw milk is a well-defined cause of listeriosis [Reference MacDonald18, Reference Linnan19], and Mexican-style cheese, such as queso fresco, is often made with raw milk. Use of pasteurized milk for making cheese should be encouraged as was done in an educational campaign conducted by Yakima County, Washington, during the 1990s [Reference Bell, Hillers and Thomas25]. Although the FDA banned the interstate sale of raw milk and of cheese made with raw milk, many states still allow these to be sold locally; these products are also sold illegally [Reference MacDonald18, 26].
Non-Hispanic pregnancy-associated case-patients were significantly more likely to report consumption of turkey breast delicatessen meat, pasta salad, butter, hot dogs, and bologna than Hispanic pregnancy-associated case-patients. Many of these food items are widely available at delicatessen counters; foods purchased from store delicatessen counters are a risk factor for sporadic listeriosis [Reference Schuchat1]. Turkey delicatessen meat, pasteurized butter, and hot dogs have all caused past outbreaks of Listeria [Reference Mead16, Reference Gottlieb17, Reference Olsen23, Reference Maijala27].
The perinatal fatality rate of 14·3% is lower than previous reports (16–34%) of perinatal listeriosis cases in the USA resulting in fetal loss or neonatal death [Reference Gellin13, Reference Mascola20, Reference Cherubin28], but 13 times higher than the overall perinatal fatality rate in the USA during 2005 [Reference MacDorman and Kirmeyer22]. A decrease in the perinatal fatality rate could be due to earlier recognition of cases as a result of increased awareness by mothers and physicians and/or a decreased rate with which physicians culture aborted tissues. Other countries report perinatal fatality rates ranging from 19% in Barcelona, Spain [Reference Nolla-Salas29] to 45% in Israel [Reference Siegman-Igra30]. Differences in perinatal fatality rates may be related to the frequencies which aborted tissues are cultured as there is great variability in incidence rates between studies and in medical centres within studies, suggesting that many cases escape diagnosis [Reference Siegman-Igra30].
Multiple births were threefold higher in infants reported to the Listeria Initiative than in the USA during 2005 [Reference Martin21]. This finding supports other evidence that pregnant women carrying multiple fetuses are at greater risk for listeriosis [Reference Mascola, Ewert and Eller8]. However, the increased proportion of multiple births seen in cases of pregnancy-associated listeriosis may be an artifact because these infants are more likely to suffer from premature birth weight and other complications, which may lead to increased culturing.
The surveillance data from the Listeria Initiative has several limitations. The Listeria Initiative is a passive surveillance system to which cases are reported. Because surveillance is limited to cases confirmed by culture, persons without access to healthcare and persons for whom illness was not confirmed by culture would not be detected. Pregnant women with no symptoms or a non-specific flu-like illness may be less likely to be identified. In addition, listeriosis cases may be missed when febrile illness is not evaluated by blood culture or if aborted fetal tissues and products of conception are not cultured. Only 20 states reported pregnancy-associated cases to the Listeria Initiative using the extended case form because use of this form is not mandatory; thus, these cases may not be nationally representative because of regional differences in demographic profiles, although reporting states represent all regions of the country, making it feasible to extrapolate our conclusions nationally. The long exposure period may make it difficult to demonstrate an association between illness and commonly eaten foods, resulting in case-patients and controls being equally likely to be exposed to commonly eaten, high-risk foods. Associations of specific food items with pregnancy-associated cases do not necessarily mean that these items are the cause of illness. The small number of pregnancy-associated cases and missing data on ethnicity make it difficult to draw statistical inferences from some comparisons. For cases where surrogates were interviewed, food history may be less reliable. The length of hospitalization for mothers and neonates was probably underestimated because not all patients had been discharged at the time of interview.
The Listeria Initiative was launched to allow for quick case-control analyses during outbreaks, since it is difficult to find suitable controls as patients with Listeria infection tend to be either pregnant or elderly, although food exposures in ill controls may be different from those of non-cases. In this analysis we used the Listeria Initiative to describe pregnancy-associated Listeria cases, leaving non-pregnancy-associated cases as the comparison group as this was the only group for which data were available. These two groups may not be comparable as non-pregnancy-associated case-patients are less likely to be Hispanic, are not exclusively female, and are often elderly or immunocompromised; therefore, this comparison, and interpretation of its results, must be viewed with caution as these two groups are quite distinct. Resulting dietary variations may be a reflection of differences in age, gender, and/or ethnicity. However, recognizing different food consumption patterns in these two high-risk groups may be important in the development of targeted educational materials.
The incidence of laboratory-confirmed invasive listeriosis decreased 24% from 1996 to 2003 [Reference Voetsch6]. During the same time period, pregnancy-associated listeriosis declined 37% [Reference Voetsch6]. This fall in the number of cases may partly be a result of efforts by food manufacturing industries and regulators to make ready-to-eat food products safer. The FDA established a zero tolerance policy for L. monocytogenes in 1985 that prohibited the presence of L. monocytogenes in ready-to-eat foods, which was adopted by the United States Department of Agriculture – Food Safety and Inspection Service (USDA-FSIS) [Reference Shank31]. In 1996, following several large outbreaks, the USDA-FSIS imposed a change in regulation by requiring that all establishments that process meat and poultry implement Hazard Analysis and Critical Control Point (HAACP) systems. After the implementation of HAACP, the proportion of ready-to-eat meat and poultry cultures tested by USDA-FSIS that tested positive for L. monocytogenes decreased [Reference Voetsch6]. However, queso fresco and other Mexican-style cheeses obtained from sources not subject to this regulation have continued to cause illness.
Pregnant women and persons at high risk for listeriosis can take precautions to lower the risk for infection. These include avoiding: hot dogs and delicatessen meats unless they are heated or reheated to 71°C; soft cheeses unless they are made from pasteurized milk; and raw, unpasteurized milk [Reference Schuchat1, Reference Schwartz2, Reference Gottlieb17, Reference Linnan19, Reference Olsen23]. Refrigerated pâtés and meat spreads, along with refrigerated smoked seafood, should also be avoided [Reference McLauchlin32–Reference Tham34]. Persons considered at risk for listeriosis should take care not to get fluid from delicatessen meat or hot dog packages on other foods or food preparation surfaces, and they should thoroughly wash their hands after handling delicatessen meats and hot dogs.
Continued education about dietary precautions for all pregnant women, especially Hispanic women and those with multiple gestations, is required to further reduce the occurrence of listeriosis. In addition, clinicians should consider the possibility of listeriosis whenever a pregnant woman presents with non-specific or flu-like symptoms because early diagnosis and treatment can be lifesaving and result in the birth of a healthy infant. Future research should be aimed at determining if multiple gestations truly increase the risk for listeriosis, and if so, understanding the mechanism(s) by which women pregnant with multiple gestations are at increased risk. Physicians should be aware of this possible association when caring for women with multiple gestations and infants born as part of a multiple birth.
DECLARATION OF INTEREST
None.





