INTRODUCTION
The burden of disease due to group A streptococcus (GAS; Streptococcus pyogenes) varies widely from place to place and population to population. The relative incidence of GAS pharyngitis appears to be higher in temperate climate regions whilst pyoderma is less common [Reference Danchin1–Reference Kumar3]. In some tropical settings and minority indigenous populations, GAS skin infection predominates and symptomatic pharyngitis is relatively rare [Reference McDonald4–Reference Ogunbi, Lasi and Lawal6]. Nevertheless, these populations may still have high rates of acute rheumatic fever (ARF) and rheumatic heart disease (RHD) [Reference Carapetis7]. Aboriginal Australians in remote tropical communities have some of the highest reported rates of ARF/RHD in the world [Reference Carapetis, Currie and Mathews8] and it has been hypothesized that skin infection plays a pathogenic role in ARF in this population [Reference Carapetis and Currie9].
The variable potential of different stains of GAS to cause post-streptococcal sequelae was observed more than 60 years ago [Reference Coburn and Pauli10, Reference Seegal and Earle11]. In the 1950s, Rammelkamp and others observed that GAS with specific M serotypes appeared to cause acute post-streptococcal glomerulonephritis (APSGN) and these were not serotypes associated with ARF [Reference Rammelkamp12]. ARF was also seen to follow GAS throat infection alone whilst APSGN occurred after either throat or skin infection [Reference Stollerman13]. This gave rise to the concept of separate skin and throat GAS types.
In the early 1970s, Widdowson and others demonstrated the presence of an M-associated protein that separated GAS into two antigenic groups (I and II) matching the known skin and throat types [Reference Widdowson, Maxted and Pinney14]. This was subsequently shown to be due to a cell surface lipoproteinase on skin GAS types that binds fibronectin and causes opacity in horse serum [serum opacity factor (SOF) or streptococcal fibronectin binding protein II – SfbII] [Reference Maxted15, Reference Rakonjac, Robbins and Fischetti16]. Known rheumatogenic M types were predominantly SOF-negative. In 1989, Bessen and co-workers differentiated two distinct classes of M protein, based on the C repeat region. Class I correlated with SOF-negative strains and contained serotypes associated with outbreaks of ARF, whereas Class II strains were associated with skin tropism [Reference Bessen, Jones and Fischetti17]. A molecular typing method for GAS was developed in the 1990s based on the nucleotides that determine the variable amino terminus of the M protein on the emm gene. M serotypes and emm types were shown to be concordant [Reference Beall, Facklam and Thompson18, Reference Bessen and Fischetti19]. Subsequently, a polymerase chain reaction (PCR)-based mapping technique further differentiated five patterns of emm and related genes; patterns A–C were associated with the throat, pattern D with the skin and pattern E was found in isolates from both throat and skin [Reference Bessen20]. With few exceptions, GAS isolates of a given emm sequence type (emmST) were found to have a predictable emm pattern type [Reference McGregor21]. An additional genetic marker for skin preference is plasminogen-binding group A streptococcal M protein (PAM) [Reference Svensson, Sjobring and Bessen22] that is found in many GAS isolates with pattern type D; it has also been associated with invasive infection [Reference McKay23].
The relationship between emmST, emm pattern type and site of GAS infection for the most part appears to hold in studies published from temperate climates and relatively low incidence settings for ARF [Reference Bessen20, Reference Dicuonzo24]. Yet, this is not the case for all tropical and high-incidence settings. For example, a study of GAS isolates from a remote Australian Aboriginal island community found that pattern types D and E were predominant, whatever the site of recovery [Reference Bessen25]. The study was limited by the paucity of throat isolates (16/141) because GAS throat carriage was so uncommon.
This study characterizes GAS isolates recovered from throat and skin swabs as part of a prospective surveillance project conducted in remote mainland Aboriginal communities with exceptionally high rates of ARF/RHD [Reference McDonald26]. The aim was to confirm the relationship between emmST, emm pattern type, PAM and SOF in these remote settings and to assess whether emm pattern type correlates with the site of GAS recovery. Finding a predominance of emm pattern types D and E would also lend support to the skin infection hypothesis in ARF.
MATERIALS AND METHODS
Source of GAS isolates
GAS isolates came from a longitudinal surveillance study of streptococcal skin infection, pharyngitis and throat carriage in three remote mainland Aboriginal communities in the tropical Top End of the Northern Territory, Australia. The communities (1, 2 and 3) were separated by at least 400 km. The aim of the study was to investigate the association of GAS isolates with ARF in these settings [Reference McDonald4]. The study protocol was approved by the local ethics committee and informed consent was obtained. Researchers then endeavoured to visit enrolled households on a monthly basis, for 2 years in the case of community 1 and for 1 year each in communities 2 and 3. Each individual was asked about sore throat (in the local language) and each throat was inspected for erythema, pus and tonsillar enlargement. Throat swabs were then taken from every person present according to a throat swab protocol, regardless of throat symptoms. Children and parents were asked about the presence of skin sores before limbs, exposed areas and the trunk were carefully inspected. Pyoderma was defined as one or more pustular, crusted, or vesicular lesions or dry and inflamed skin ulcers >1 cm in diameter. Non-infected insect bites and non-inflamed partly healed pyoderma lesions were excluded [Reference McDonald4]. Swabs were taken from any skin sores. Additional clinical and demographic information was also collected.
Because we were unable to obtain blood samples for serological testing, individuals were considered to be carrying GAS in the throat if GAS was recovered from the throat swab and they were asymptomatic at the time.
Recovery and identification of GAS isolates
Culture methods have been described in more detail elsewhere [Reference McDonald26]. Plates were incubated at 37°C in 5% CO2 and examined after 24 h and 48 h. Large colony-forming beta-haemolytic streptococci (BHS) were subcultured for identification using a Streptococcal Grouping kit (Oxoid Diagnostic Reagents, England, UK). Isolates were then stored in tryptone soya broth (Oxoid) with 15% glycerol at −70°C.
emm sequence type determination
The procedure for emm sequence typing followed that recommended on the Centers for Disease Control and Prevention (CDC) website [27] with minor modifications. We used InstaGeneTM matrix (Bio-Rad, Hercules, CA, USA) for DNA sample preparation; 20 colonies were harvested directly from culture plates and re-suspended in 200 μl. All other steps were according to manufacturer's instructions. PCR was conducted in 50 μl reaction volumes with 5 μl of the InstaGeneTM preparation, 10 nmol dNTPs, 25 pmol each of primers 1 and 2, 2·5 U Taq (Qiagen, Hilden, NRW, Germany), 5 μl 10× Taq buffer, sdH2O to 50 μl. PCR clean-up was done using QIAquick 96 PCR purification kit (Qiagen), eluting in 60 μl sdH2O.
For the sequencing reaction undiluted BigDye terminator version 3.1 (Applied Biosystems, Foster City, CA, USA) premix in half volume (10 μl) with 2 μl purified PCR product, 12·5 pmol emmseq2, 4 μl premix and 3·5 μl sdH2O was used and the reaction program was as per protocol. Clean up was achieved by adding 10 μl sdH2O and using DyeEx96 (Qiagen). The product was desiccated and sent to a commercial sequencing facility for separation. For sequence analysis, electropherograms were viewed with DNAstar Seqman software (Madison, WI, USA) and sequences were blasted against the CDC Streptococcus pyogenes emm sequence database [27]. Novel emm sequences were submitted to the administrator for assignment.
emm pattern type determination
The emm pattern type was determined according to the protocol described by Bessen et al. [Reference Bessen25], with a restricted set of primers as shown in Table 1.
Table 1. Primer set used for emm pattern typing [Reference Bessen25]

Presence of PAM and SOF
PAM-positive emmST were differentiated by identifying sequences with homology to the plasminogen-binding A repeats of PAM within the deduced amino-acid sequence of the translated emm sequence data [Reference Svensson, Sjobring and Bessen22, Reference McKay23]. Information regarding the presence of the SOF phenotype for each emm sequence type was obtained from the CDC website and published sources [27, Reference Johnson28].
Data analysis
Data were analysed using stata 8 (Stata Corporation, College Station, TX, USA). Relative risks (RR) for emm pattern types were calculated by comparing the proportions for each site of GAS recovery and confidence intervals (CI) were calculated by standard methods.
RESULTS
Surveillance and recovery of GAS isolates
Forty-nine households and 1173 people were enrolled. There were 531 household visits and 4842 consultations. Some of the epidemiological data have been documented in a previous report; symptomatic pharyngitis was extremely rare in study children and pyoderma was common [Reference McDonald4]. Three hundred and fifty GAS isolates were available for molecular typing, 222 from the throat and 128 from pyoderma lesions. All isolates were emm sequence typed. The first 244 GAS isolates, 150 throat and 94 skin isolates, were also emm pattern typed (Fig. 1).

Fig. 1. Flow diagram of GAS isolates: determination of emm pattern type.
Long-term throat carriage of a specific GAS emmST was uncommon and there were only six people in the study who had the same emmST recovered from the throat on more than one occasion (providing an extra nine isolates). If GAS of a specific emmST was recovered from multiple skin sites from a person on the same day, they were not counted as multiple isolates. However, one child had emm100 recovered from skin sores for two consecutive months (providing one extra isolate). Otherwise we did not recover GAS from skin sores in a serial manner from anyone else. As a result 10 GAS isolates were removed from the analysis (Fig. 1). There were eight instances where GAS was recovered from the skin and the throat from one individual at the same visit; on four occasions they had a different emmST in the throat from that on the skin and four had the same emmST at both sites. These were left in the analysis. On 61 occasions more than one person in a household was colonized with a specific emmST at the same time or at a subsequent visit.
Correlation of emm sequence type, emm pattern type and site of recovery
Consistent with published data [Reference McGregor21, Reference Bessen25], we found 100% concordance between emm sequence type and emm pattern type in the first 244 GAS isolates from this study. We were therefore confident about predicting the emm pattern type for the remaining 106 GAS isolates where we knew the emm sequence type (Fig. 1). Summary data for emm sequence type, emm pattern type and site of recovery are shown in Table 2. The combined emm pattern data for skin and throat isolates are shown in Figure 2. Of 213 isolates recovered from the throat, 61 were pattern types A–C (29%) whilst 16/127 skin isolates were types A–C (13%) (RR for throat 2·3, 95% CI 1·4–3·8). Pattern type D accounted for 67/127 (53%) skin isolates and 51/213 throat isolates (24%) (RR for skin 2·21, 95% CI 1·7–3·0). Pattern type E was also more common in throat isolates (101/213, 47%), than skin isolates (44/127, 35%) (RR for throat 1·4, 95% CI 1·1–1·8). GAS was recovered from the throat of only two people in the study with symptomatic pharyngitis (both adults); the emm sequences types were 11 and 87 (both emm pattern type E).
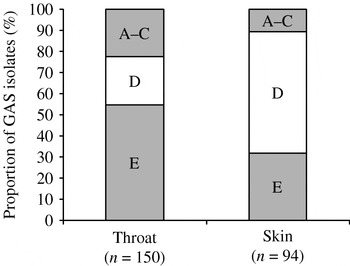
Fig. 2. Percentage of emm pattern types by site of recovery.
Table 2. emm sequence types and emm pattern types from three remote communities

Outbreak of emm55
The data were skewed by an outbreak during February 2005 when the prevalence of pyoderma in children of community 3 jumped to 18% compared to the median point prevalence of 6·1%. It coincided with the sudden appearance of GAS emm55 (pattern types A–C) in both skin sores (n=5) and throat (n=18). The strain had not previously been seen in the study. There was no discernible increase in the incidence of sore throat or proven GAS pharyngitis, but there was a subsequent community outbreak of APSGN [Reference Binns, Markey and Krause29]. The same emm sequence type (emm55) then appeared in community 1 during April 2005 (skin=2 and throat=15); it was not accompanied by a detectable increase in pyoderma or pharyngitis, but it did appear to precipitate another outbreak of APSGN (Northern Territory Centre for Disease Control, Darwin, unpublished data). Up to the time of the appearance of emm55, pattern types A–C had accounted for only 12/179 (6·7%, 95% CI 4–11) of GAS isolates. In the first 6 months of 2005, types A–C accounted for 65/162 (40%, 95% CI 33–48) of GAS isolates, 72% (47/65) of those were emm55. Overall, this sequence type accounted for 47/61 (77%) of pattern A–C throat isolates and 14/16 (88%) of A–C skin isolates.
Gradation of tissue tropism
Thirteen of the most common emm sequence types (with >10 isolates) and site of recovery are shown in Figure 3, together with PAM and SOF patterns. A transition from pattern types A–C, through pattern type E, and then to pattern type D can be seen as the GAS isolates move from throat tropic to more skin tropic. One exception is the outbreak strain, emm55. Three of the five most common pattern D isolates were PAM positive and SOF was positive in all six of the common pattern E types. Pattern A–C types (emm1 and emm55) were negative for both PAM and SOF.

Fig. 3. The most common GAS emm sequence types (>10 isolates) showing the percentages recovered from the throat and from the skin together with emm pattern type. PAM, Presence of the plasminogen-binding group A streptococcal M protein genotype; SOF, serum opacity factor.
DISCUSSION
The findings of this study confirm that emm pattern type can be predicted by emm sequence type in GAS isolates from these remote communities. This matches the findings of McGregor et al. who studied 495 GAS isolates collected from many parts of the world [Reference McGregor21] including several tropical countries. The emm gene codes for M protein, a key anti-phagocytic virulence factor that forms the basis of M serological typing and is responsible for evoking serotype-specific host immunity. For the most part, specific emm types are clonally related, when compared by multilocus sequence typing (MLST) using bacterial housekeeping genes, although there are some exceptions where one MLST sequence type is associated with more than one emm sequence type [Reference McGregor21]. This mainly occurs in skin types (emm pattern D) and suggests recombinatorial replacement of emm type. Such recombination may be most likely to occur at skin sites, such as in pyoderma, where multiple GAS strains can be present together and accompanied by selective pressure from the host immune response.
The distinction between types associated with throat and skin appears to be most marked in temperate regions of the northern hemisphere. M serotypes or emm sequence types corresponding to pattern types A–C or E predominate in throat isolates from the United Kingdom, continental Europe and the United States [Reference Martin2, Reference Dicuonzo24, Reference Colman30, Reference Fiorentino31]. By contrast, pyoderma was found to be the dominant manifestation of GAS infection in a remote Aboriginal island community and was mainly attributable to pattern types D (43%) and type E (40%); one reason may be that skin isolates outnumbered throat isolates by 10:1 [Reference Bessen25]. A–C was the most abundant pattern type in throat isolates, but the numbers were small with only three emmST identified. This study covered a much larger population and produced a high proportion of throat isolates, providing the GAS emm pattern type distribution in widely separated mainland Aboriginal communities that was similar to that described in the remote island population. It is reasonable to suggest that this emm pattern type distribution is likely to be the norm rather than the exception across the region.
A previous US study of serological response had shown that patients with ARF have an elevated response to the specific class I epitope but little response to class II epitope [Reference Bessen32]. In Australian Aboriginal populations, raised levels of antibodies to class I and class II epitopes have not been associated with any specific disease but probably indicate the burden of GAS exposure [Reference Brandt33]. In this setting, it has been suggested that the high rates of impetigo in childhood engender non-type specific protective immunity against later throat colonization and pharyngitis. This has yet to be proven.
A similar picture to that seen here has been observed in a study from Ethiopia where 78 different emm sequence types were found in 217 GAS isolates [Reference Tewodros and Kronvall34]. Pattern type E was responsible for 38% of isolates, pattern D for 28% and patterns A–C for 18%. Patterns A–C accounted for 3% of skin isolates and 24% of throat isolates. Pattern D was also responsible for 28% of GAS pharyngitis and 4/7 of the isolates associated with ARF. Likewise pattern types D and E dominate in populations of north and south in India, although the rates of throat and skin infection are markedly different; the south Indian population has high rates of pyoderma and the north Indian has high rates of pharyngitis [Reference Sharma and Sriprakash35, Reference Dey36].
Despite prevalence rates of RHD >25/1000 in the three communities of this study (Northern Territory Rheumatic Heart Disease Prevention Program, unpublished data), pyoderma was the outstanding manifestation of GAS infection in children and emm pattern types D and E of GAS predominated. This study had a much higher proportion of throat isolates (222/350, 63%) than did a previous study conducted among Aboriginal people living on a remote island (16/141, 11%) [Reference Bessen25] and provides a broader view. The data suggest that the background rate of pattern types A–C in the three mainland communities was <7% but the proportion was subsequently inflated by outbreaks in the two large communities of emm55 having emm pattern type A–C. Ironically, the outbreaks involved GAS skin infections (in community 3), not pharyngitis, and were also accompanied by outbreaks of APSGN rather than ARF. GAS M serotype 55 has previously been associated with similar outbreaks of APSGN in the Top End of the Northern Territory, Trinidad and elsewhere [Reference Kearns, Evans and Krause37–Reference Dillon39]. Another emm pattern type A–C, M/emm 12, has been associated with outbreaks of APSGN [Reference Majeed40] and raises additional questions about the specificity of emm pattern as a predictor of disease.
It has previously been pointed out that emm pattern type is an imperfect marker of tissue tropism and does not preclude infection at other sites [Reference Bessen25]. Indeed, the factors that determine tissue preference seem to be complex; they include SOF and PAM for the skin [Reference Maxted15, Reference Svensson, Sjobring and Bessen22] and maltodextrin utilization conferring the ability to survive in saliva for the throat [Reference Shelburne41]. Much remains a mystery. In this study (Fig. 3), PAM provided some indication of tissue preference; SOF provided none. The three most throat tropic emmST were exclusively isolated from the throat, whereas even the most skin tropic emmST were able to colonize the throat. Colonization of the throat following skin infection is not uncommon [Reference Anthony42]. These results suggest that throat colonization is the default condition and that the ability to cause skin infection is probably due to the presence of one or more skin tropic factors that are absent in throat strains.
Our study reaffirms that where ecological and epidemiological factors predispose to skin infection, as occurs in overcrowded tropical environments with poor hygiene conditions [Reference Currie43], emm pattern types D and E prevail and even account for the majority of throat GAS isolates. Pyoderma is likely to remain the predominant GAS infection in remote tropical Australian Aboriginal communities in the absence of effective interventions [Reference Currie43]. There is evidence for extensive genetic recombination between GAS strains and this is more likely to occur where there is a high burden of GAS colonization and infection, especially where more than one strain occupies the same site [Reference Kalia44]. Despite this recombination, our study confirms concordance between emm sequence type and emm pattern type when compared with the published data [Reference Brahmadathan5, Reference McGregor21, Reference Bessen25]. We therefore also conclude that emm pattern typing in these tropical settings, which is labour intensive and difficult to interpret, offers no useful information in addition to that provided by emm sequence typing when investigating the epidemiology of GAS.
ACKNOWLEDGEMENTS
We thank the families in the communities, the Aboriginal research officers and community health centre staff for their participation and support. We are also grateful to Dr Bernard Beall of the Centers of Diseases Control and Prevention (Atlanta) for his helpful advice. Hamish Cameron assisted with the emm sequence and pattern typing.
DECLARATION OF INTEREST
None.









