INTRODUCTION
Tuberculosis (TB) continues to be a major cause of illness and mortality worldwide [1]. HIV-infected individuals have a high risk of developing clinical TB, and HIV has fuelled the TB epidemic in sub-Saharan Africa. TB patients often suffer from malnutrition, especially micronutrient deficiencies [Reference Semba, Darnton-Hill and de Pee2, Reference van Lettow, Fawzi and Semba3]. Studies have shown that TB patients have a high prevalence of deficiencies in vitamins A, B-complex, C, E, and selenium [Reference Semba, Darnton-Hill and de Pee2].
Host defence against Mycobacterium tuberculosis infection requires cell-mediated immunity, which involves coordination of macrophages and T-cell lymphocytes. Patients with a dual burden of mycobacterial infection and micronutrient deficiencies may have impaired cell-mediated immune response. However, a previous review concluded that there is insufficient evidence for a beneficial effect of multiple micronutrient supplements on clinical outcomes in TB patients [Reference Sinclair4]. We have previously reported that micronutrient supplementation significantly decreased the risk of early TB recurrences in Tanzania [Reference Villamor5]. Understanding potential mechanisms mediating such beneficial effects is important. In this paper, we examine the effect of micronutrient supplements on lymphocyte proliferation response to mycobacteria or T-cell mitogens, markers of cell-mediated immune function, in a randomized trial conducted on patients with pulmonary TB.
METHODS
Study design and population
From April 2000 to April 2005, adults with pulmonary TB were enrolled in a randomized, double-blind, placebo-controlled trial to examine the effect of micronutrient supplements on TB treatment outcomes and mortality. Details of the study design have been published previously [Reference Villamor5]. Participants were recruited from five outpatient TB clinics in Dar es Salaam, Tanzania. Eligible participants were men and non-pregnant women aged between 18 and 65 years who had at least two positive sputum smears for acid-fast bacilli (AFB). Patients with a Karnofsky performance score of <40% or haemoglobin concentration of <7·0 g/dl were excluded from the study. HIV testing was conducted using two sequential enzyme-linked immunosorbent assays (ELISA) in an alternative confirmatory strategy and discrepant results were resolved by Western blot assay. Participants were stratified by HIV status and randomly assigned to receive a daily dose of micronutrients (5000 IU retinol, 20 mg vitamin B1, 20 mg vitamin B2, 100 mg niacin, 25 mg vitamin B6, 50 μg vitamin B12, 500 mg vitamin C, 200 mg vitamin E, 0·8 mg folic acid, 100 μg selenium) or placebo at the time of initiation of TB treatment. As recommended by the Tanzania National TB and Leprosy Programme at the time of the study, patients received a daily combination of rifampicin, isoniazid, pyrazinamide, and ethambutol at the health facility under direct observation of a healthcare worker for the first 2 months. In the subsequent 6 months, patients self-administered isoniazid and ethambutol at home. Antiretroviral therapy was not available to most HIV-positive participants at the time of the study in Tanzania.
At the baseline visit, trained research assistants obtained information about demographic and socioeconomic characteristics. Follow-up was conducted every month at the study clinics where research nurses inquired about the health of the patient. A physician carried out a complete physical examination every 3 months. Sputum for AFB smears and cultures was collected. A blood sample was obtained to measure haemoglobin concentrations and CD4+, CD8+, and CD3+ T cells using the FACScount or FACScan systems (BD Biosciences, USA). HIV viral load was also measured using the Roche Amplicor v. 1.5 assay (Roche Diagnostics, USA). The HIV disease stage was assessed according to the World Health Organization (WHO) staging system [6].
The study protocol was approved by the Institutional Review Boards at the Muhimbili University of Health and Allied Sciences, the Tanzanian National AIDS Control Program, and the Harvard School of Public Health. All patients provided written informed consent to participate in the trial.
Assessment of lymphocyte proliferation assay response
In vitro lymphocyte proliferation is frequently used as a marker for cell-mediated immune function. Lymphocyte proliferation was measured by [3H]thymidine incorporation after stimulation with T-cell mitogens or TB antigens using the whole-blood culture method. For each patient, 5 ml blood was collected into heparin-containing Vacutainers for the whole-blood assay. The blood was diluted at 1:10 with complete Roswell Park Memorial Institute Medium (RPMI 1640 culture medium supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mmol/l l-glutamine, 25 mmol/l Hepes). Diluted blood was distributed into 96-well tissue culture plates, and then stimulated with concanavalin A (ConA) at 5, 25, and 50 mg/l, phytohaemagglutinin (PHA) at 2, 10, and 50 mg/l, or purified protein derivatives (PPD) at 2, 5, and 20 mg/l. The plates were incubated for 72 h at 37°C in a 5% CO2 humidified atmosphere. Cultures were pulsed with [3H]thymidine during the final 4 h of incubation. At the end of incubation, the plates were first frozen at –70°C overnight and then stored at –20°C before harvest. After 1 h of incubation at 37°C in trypsin/EDTA solution, cells were harvested using an automatic harvester. Cell proliferation was quantified as the amount of [3H]thymidine incorporation into DNA as determined by a liquid scintillation counter. The results of in vitro stimulation of lymphocytes were reported as counts per minute (cpm). We report results generated with use of the optimal concentration for each stimulant: 25 mg/l ConA, 10 mg/l PHA and 5 mg/l PPD. Similar results were found when other concentrations were used for stimulation (data not shown).
Statistical analysis
Of 887 adults with pulmonary TB (416 HIV-negative and 471 HIV-positive patients) enrolled in the trial, at least one measurement of lymphocyte proliferation at 2 or 8 months was made for 423 patients (267 HIV-negative and 156 HIV-positive patients). Due to logistics issues, lymphocyte proliferation could not be measured in all participants, especially HIV-positive patients. We compared the distribution of baseline characteristics between treatment groups within the strata of HIV status by using the Wilcoxon rank sum test for continuous variables and the χ 2 test for categorical variables. An intention-to-treat analysis was performed. To examine the effect of micronutrient supplements on lymphocyte proliferative responses, we conducted an analysis of parallel mean response profiles with adjustment for baseline using generalized linear models for repeated measurements [Reference Fitzmaurice, Laird and Ware7]. The robust empirical variance structure was used. All statistical analyses were conducted using SAS v. 9.1 (SAS Institute Inc., USA).
RESULTS
We examined the characteristics of 423 TB patients with the measurement of lymphocyte proliferative responses by treatment arms within strata of HIV status (Table 1). Patients' baseline characteristics were similar between micronutrient and placebo groups. We found no significant effect of micronutrient supplements on lymphocyte proliferative responses to T-cell mitogen (10 mg/l PHA) or mycobacterial antigens (5 mg/l PPD) during TB treatment in HIV-negative and HIV-positive TB patients (Table 2). Of HIV-negative TB patients, the micronutrient group tended to show higher proliferative responses to T-cell mitogen (25 mg/l ConA) than the placebo group (P = 0·04), although the effect was limited to 2 month.
Table 1. Baseline characteristics of tuberculosis patients
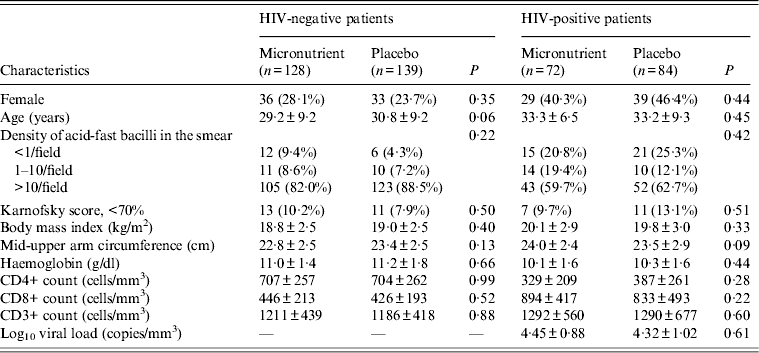
Values are mean ± s.d., otherwise n (%).
The Wilcoxon rank sum test was used to compare continuous variables and the χ 2 test was used for categorical variables.
Table 2. Effect of micronutrient supplements on lymphocyte proliferation response during tuberculosis treatment
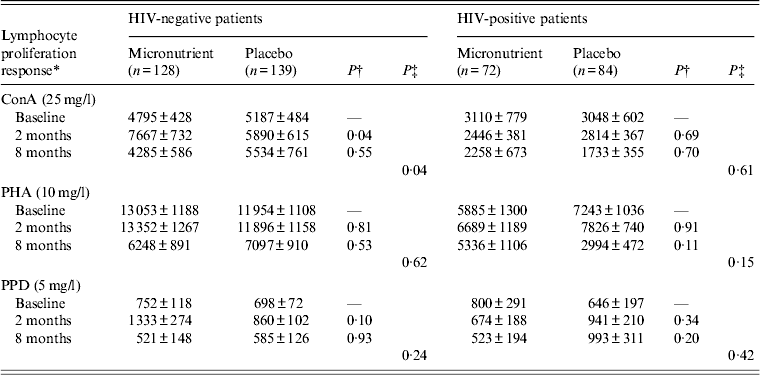
Values given are mean ± s.e.
* Whole blood was stimulated in vitro with T-cell mitogens, concanavalin A (ConA) and phytohaemagglutinin (PHA), or mycobacterial antigens (purified protein derivative; PPD) and measured by [3H]thymidine incorporation. Values are expressed as counts per minute (cpm).
† Treatment effects at 2 or 8 months. P value was obtained from an analysis of parallel mean response profiles with adjustment for baseline response. We used the general linear model for repeated measurements with the robust empirical variance.
‡ Overall treatment effects.
DISCUSSION
We found no overall effects of supplementation with micronutrients at multiples of the recommended dietary allowance on lymphocyte proliferative responses to T-cell mitogens or mycobacterial antigens in HIV-negative and HIV-positive TB patients. Of HIV-negative TB patients, the micronutrient group tended to show higher proliferative responses to ConA than the placebo group, although the clinical relevance of this finding is not readily notable. In this trial, micronutrient supplementation was found to significantly decrease the risk of early TB recurrence by 45% in all TB patients and increased CD4+ cell counts in HIV-negative patients [Reference Villamor5]. Supplementation also reduced the incidence of peripheral neuropathy by 57% in all patients. Although unexpected, our overall lack of effects on lymphocyte proliferation could be due to a number of reasons. We only assessed these immune parameters twice, at 2 and 8 months, and detection of an effect may have required earlier assessments, particularly during the first 2 months of the anti-TB treatment period when active clearance of M. tuberculosis occurs. Furthermore, we did not measure other markers such as the production of cytokines, including interferon-γ and tumour necrosis factor-α, which may have more fully captured the effects of micronutrient supplementation. The lack of findings could also be due to measurement error during laboratory assays thereby biasing any potential association towards the null. Our results are unlikely to be due to lack of adherence, because our study had high follow-up rates and high compliance with the study regimen. Additionally, adequate dosages of micronutrient supplements were provided in this trial.
Contrary to our findings, previous studies have addressed the important role of vitamins on immune responses to mycobacterial infection. Vitamin A plays a crucial role in the cell-mediated response and the lymphocyte proliferative response is up-regulated by retinoic acid receptors [Reference Wintergerst, Maggini and Hornig8, Reference Karyadi9]. Antioxidants, such as vitamins C and E, and selenium may suppress reactive oxygen species produced by mycobacteria and prevent tissue injury and inflammation. Oxidative stress and excessive inflammatory responses may suppress T-cell function. An in vitro study using peripheral blood mononuclear cells from TB patients found that vitamin E enhances lymphocyte proliferation in response to PPD [Reference Hernandez10]. Vitamin B6 deficiency has been associated with decreased lymphocyte responsiveness to T-cell mitogen and decreased natural killer cell activities [Reference Villamor5, Reference Baum11, Reference Cheng12].
Several limitations should be noted. Supplements in our study did not include zinc or vitamin D, and these nutrients play important role in improving immune functions and TB treatment outcomes [Reference Sinclair4]. Vitamin D induces antimycobacterial activity in macrophages, and observational studies have shown that low serum vitamin D levels increase the risk of active TB [Reference Nnoaham and Clarke13]. It is uncertain whether relatively low lymphocyte proliferative responses in our study compared to previous studies may be due to the study population or laboratory method. We used the whole-blood culture method instead of the isolated peripheral blood mononuclear cells method, because whole-blood culture requires only a small amount of blood and is much less labour intensive. At the time of the study, antiretroviral therapy was not available to most HIV-positive participants in Tanzania. Therefore, our findings need to be confirmed under concomitant treatment with antiretroviral therapy in HIV-positive patients.
In conclusion, we found no overall effects of micronutrient supplementation on T-cell-mediated immune responses in patients treated for pulmonary TB. Micronutrient supplements have been found to reduce TB recurrence in TB patients and the role of nutritional intervention in this vulnerable population remains an important area of research [Reference Semba, Darnton-Hill and de Pee2]. Additional studies are needed to understand the interactions between nutrition, immune function, and clinical outcomes in TB patients.
ACKNOWLEDGEMENTS
We thank the patients who participated in this study. We also thank the study physicians, nurses, laboratory and administrative staff at Muhimbili University of Health and Allied Sciences and the TB clinics in Dar es Salaam, Tanzania. This study was supported by the National Institute of Allergy and Infectious Diseases (UO1 AI 45441-01) and USDA contract 58-1950-7-707.
DECLARATION OF INTEREST
None.






