INTRODUCTION
Studies on bacteraemia have identified numerous prognostic predictors, such as age, gender, comorbidity, bacterial species, nosocomial acquisition, and focus [Reference Young, Mandell, Bennett and Dolin1]. Owing to several of the different predictors being interrelated (e.g. older age and higher comorbidity) the prognostic impact of individual predictors is difficult to quantify. One of the main concerns is the prognostic impact of invasion of the blood stream per se, and while attention to bacterial species is plentiful [Reference Young, Mandell, Bennett and Dolin1, Reference Weinstein2] much less attention has been given to magnitude of bacteraemia. Mainly older studies reported that higher magnitude of bacteraemia was associated with increased mortality [Reference Tilghman and Finland3–Reference Schønheyder11]. However, these studies had a number of limitations, including small numbers of patients, follow-up time limited to the hospital admission in question, use of cumbersome blood culture (BC) methods with low sensitivity, and no adjustment in multivariate analyses for potentially important covariates.
In this population-based study we made use of our routine BC practice in which 30 ml blood is equally distributed into three bottles. The number of positive bottles in the first positive BC set may be a semi-quantitative index of magnitude of bacteraemia. Our aim was to examine if this index was a predictor of mortality during a 1-year follow-up period in patients with first-time mono-microbial bacteraemia, taking other known predictors into consideration.
METHODS
Setting
We conducted the study in North Jutland County, Denmark, covering the 9-year period 1996–2004. We included all adults (aged ⩾16 years) with a first-time episode of mono-microbial bacteraemia (excluding fungemia), restricting the study to the patient's first positive BC set.
The Danish health-care system is financed through the tax system and provides care free of charge for all residents. Acutely ill patients are admitted to the nearest hospital in their county of residence. During the study period the county had an average population of 494 004 [12], served by eight public hospitals. Bacteriological services for the entire county were provided by the Department of Clinical Microbiology, Aalborg Hospital.
Record linkage
All Danish residents have a personal identification number, which permits accurate linkage among registries [Reference Pedersen13]. The Danish Civil Registration System contains daily updated records on the vital status of all Danish residents, including date of emigration or death, which allowed us to ascertain patient status at follow-up.
North Jutland County Bacteraemia Research Registry
We identified patients with bacteraemia in the North Jutland County Bacteraemia Research Registry. Since 1992, all bacteraemic and fungemic episodes have been prospectively recorded in this registry, which contains data on microbial pathogens, the patient's successive bacteraemia number (first, second, etc.) since 1981, number of positive bottles in each BC set (from 1996), infection acquisition, infection focus, and ward on the date of venepuncture [Reference Schønheyder and Højbjerg14].
Bacteraemia was defined as bacterial growth in a BC set in which the isolated pathogen was given aetiological significance based on clinical and microbiological criteria [Reference Weinstein15]. Coagulase-negative staphylococci, Bacillus spp., Corynebacterium spp., and Propionibacterium acnes were regarded as contaminants unless isolated from two or more separate BC sets.
Blood culture procedures
The BC system BacT/Alert (bioMérieux, Marcy l'Etoile, France) was used throughout the study period. A BC set comprised two aerobic bottles and one anaerobic bottle; only standard bottles were used from 1996 to 1998, after which one aerobic standard bottle was substituted by an aerobic FAN bottle [Reference Weinstein16]. The BC bottles were inoculated at bedside by a trained phlebotomist with a nominal volume of 10 ml blood each. The bottles were monitored up to 6·7 days after incubation. Bottles with growth were unloaded at fixed hours (08:00, 11:00, 14:00 and 20:00 hours) and examined by direct microscopy, including wet mount and Gram stain [Reference Søgaard, Nørgaard and Schønheyder17], and subcultured on bacteriological media as appropriate. From March 2000 to February 2002 all bottles were weighed, and amongst the 1127 weighed BC sets included in this study there were no weight differences between BC index 1 (BCI 1), BCI 2, and BCI 3 (data not shown).
Speciality
The patient's ward on the date of venepuncture was recorded as medical, surgical, intensive care unit (ICU), or miscellaneous (mainly mixed surgical/medical wards in smaller hospitals).
Acquisition of infection
The infection was defined as community-acquired or nosocomial according to CDC criteria [Reference Garner18] in addition to a ‘health-care related’ group with hospital contact within 30 days up to the bacteraemic episode [Reference Friedman19].
Infection focus
A focus was documented microbiologically or clinically as described previously [Reference Pedersen and Schønheyder20] and classified into urinary tract, respiratory tract, abdominal and/or hepatobiliary system, miscellaneous (mainly the circulatory system, central nervous system, bones, joints, or soft tissues), or unknown.
Comorbidity
We used the Hospital Discharge Registry of North Jutland with its International Classification of Diseases (ICD) system (ICD-8 in 1977–1994 and ICD-10 thereafter) [Reference Andersen21]. We identified selected major comorbid diseases recorded in the 5-year period prior to the bacteraemia. We classified comorbidity using the Charlson index, which includes 19 major disease categories (e.g. cardiovascular diseases, cancer, and diabetes) and assigns scores to each of these (with higher scores associated with more severe disease categories) [Reference Charlson22].
Statistical analysis
The analytical unit was the patient's first positive BC set and the baseline date was the date of sampling this BC set. The primary exposure was the number of positive BC bottles [BCI 1 (reference), BCI 2, or BCI 3] in this BC set. Outcome was death within 1 year after the baseline date.
The Charlson index was categorized (scores 0, 1–2, >2). For descriptive purposes, we categorized age into three groups (16–64, 65–80, >80 years), but kept it as a continuous covariate in the analyses.
Initially, we analysed the data separately for the periods 1996–1998 and 1999–2004 to evaluate if the replacement of one aerobic standard bottle with an aerobic FAN bottle in 1999 changed the relation between exposure and outcome. As this was not the case, the two periods were merged in the further analyses.
We cross-tabulated the BCI and computed Kaplan–Meier mortality curves, overall and in subgroups. These revealed that differences in mortality occurred within certain time periods, which led to splitting the follow-up time into three time periods (0–7, 8–30, and 31–365 days).
We used Cox's proportional-hazards regression analysis to estimate risk of mortality, calculated as mortality rate ratios (MRR) and 95% confidence intervals (CI) within each of the three time periods; analyses were both crude and adjusted for potential covariates including comorbidity, age, gender, speciality, infection acquisition and focus. We repeated Cox's regression analyses within major subgroups (gender, comorbidity, age, speciality, acquisition of infection, focus, bacterial groups and predominant species). The assumption of proportional hazards in the models was assessed graphically and found appropriate within all time periods in the model adjusted for comorbidity and age, which we selected as our final model.
The excess number of deaths amongst BCI 3 patients compared to BCI 1 patients was assessed from the standardized cumulated mortality (standardized to the comorbidity and age distribution as in BCI 1).
The software program Stata/SE 9.2 for Windows (Stata Corporation, College Station, TX, USA) was used for all analyses.
Ethical considerations
The study was conducted according to the guidelines of the regional scientific ethics committee for use of clinical and laboratory data and approved by the Danish Data Protection Agency (Record no. 2006-41-6969).
RESULTS
A total of 6406 patients were included in the study and 1-year follow-up was possible for all patients but one.
BCI distribution
Facultatively anaerobic bacteria were involved in 92·5% of all the bacteraemias (data not shown). The overall distribution was U-shaped, with 31·1%, 18·3%, and 50·6% belonging to BCI 1, BCI 2, and BCI 3, respectively (data not shown). Deviations from this distribution were minor for most major subgroups. Gram-positive bacteria (in particular Staphylococcus aureus and Streptococcus pneumoniae) and the miscellaneous focus group had a higher proportion of BCI 3 sets relative to BCI 1 sets. Gram-negative bacteria and the abdominal/hepatobiliary focus groups had a higher proportion of BCI 1 sets relative to BCI 3 sets.
Kaplan–Meier mortality curves
Overall, BCI 3 patients had higher mortality compared to BCI 1 and BCI 2 patients, a difference which occurred within the first 30 days, after which the mortality curves tended to be parallel (Fig. 1a). This trend was especially observed for medical patients (Fig. 1b), whilst for surgical patients higher mortality associated with BCI 2 and BCI 3 were especially observed beyond 30 days (Fig. 1c).

Fig. 1. Kaplan–Meier mortality curves, related to blood culture (BC) index (number of bottles with bacterial growth in the patient's first-time three-bottle BC set). —, BC index 1; - - - -, BC index 2; – · – · –, BC index 3. (a) All patients (n=6406); (b) medical patients (n=3842); (c) surgical patients (n=1691).
BCI 3 vs. BCI 1
The overall mortality risk estimates were consistent with the Kaplan–Meier mortality curves. Adjusted mortality risk estimates were not substantially different from the crude ones (Table 1). BCI 3 predicted higher risk of mortality up to day 30 (days 0–7: 1·30, 95% CI 1·10–1·55; days 8–30: 1·37, 95% CI 1·12–16·8), but not thereafter (days 31–365: 1·07, 95% CI 0·94–1·22). This trend was consistent in several subgroups [males, patients with comorbidity, patients aged 65–80 years, medical patients, and patients with community-acquired bacteraemias, a urinary tract focus, or Gram-negative bacteria (especially Enterobacteriaceae)]. In other subgroups the excess mortality was observed within 7 days, but not in the 8- to 30-day period [patients aged 16–64 years, and patients with a health-care-related acquisition of infection, a respiratory tract focus or an unknown focus, or Gram-positive bacteria (especially S. pneumoniae)]. Beyond 30 days, BCI 3 predicted higher mortality in patients with an intermediate level of comorbidity, patients aged 16–64 years, surgical patients, patients with a urinary tract focus, and patients with E. coli bacteraemia. Finally, the BCI was a poor predictor of mortality in patients without comorbidity, ICU patients, in patients with a nosocomially acquired bacteraemia, and in patients with an abdominal/hepatobiliary or a miscellaneous focus, Staph. aureus, and other bacteria.
Table 1. Mortality rate ratios (95% confidence intervals) for blood culture index (BCI)Footnote * 3 with BCI 1 as reference. Estimates were obtained from Cox's proportional-hazards regression analyses and adjusted for Charlson comorbidity index and age, unless otherwise indicated
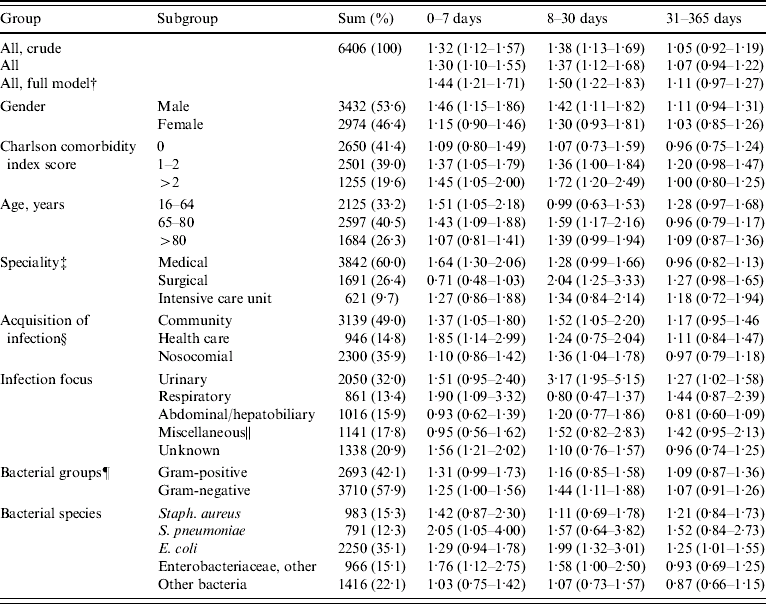
* Number of bottles with bacterial growth in the patient's first-time three-bottle BC set.
† Model adjusted for Charlson comorbidity index, age, speciality, acquisition of infection, infection focus, and gender.
‡ ‘Miscellaneous’ (cf. text) omitted (n=252).
§ ‘Unknown’ omitted (n=21).
∥ Mainly the circulatory system, central nervous system, bones, joints, or soft tissues.
¶ Patients with unknown bacterial species omitted (n=3).
Results for patients with a respiratory tract focus and S. pneumoniae were highly correlated. We therefore repeated the analyses for S. pneumoniae with the inclusion and exclusion of a respiratory tract focus and a respiratory tract focus with the inclusion and exclusion of S. pneumoniae. Mortality risk estimates in the 0- to 7-day period remained high in patients with a respiratory tract focus and with other bacteria than S.pneumoniae, but approached 1 in the group with S. pneumoniae and other foci than the respiratory tract (data not shown), which indicated that the focus exerted the main impact.
Similarly, a urinary tract focus and E. coli were correlated. For the combination of E. coli and urinary tract focus mortality risk estimates increased beyond 7 days, whilst they were close to 1 in the two other groups; thus we found no further evidence as to what mainly contributed to these results (data not shown).
BCI 2 vs. BCI 1
Few differences in mortality risk estimates were encountered between BCI 1 and BCI 2 (data not shown), except at 0–7 days for ICU patients (MRR: 1·74, 95% CI 1·08–2·79), a respiratory focus (2·28, 95% CI 1·23–4·23) and S. pneumoniae (2·33, 95% CI 1·13–4·81) and at 8–30 days for surgical patients (2·02, 95% CI 1·14–3·60).
Excess number of deaths
If the BCI 3 patients had the same standardized cumulated mortality as the BCI 1 patients 1163/3239 (95% CI 1098–1228) would be expected to die, however, 1299 actually died (95% CI, 1247–1351), which is 136 additional deaths (95% CI 19–253 deaths). Of note, the 4·2% excess mortality was comparable to the standardized cumulated mortality difference of 3·3% between medical (35·9%, 95% CI 34·5–37·4) and surgical (32·6%, 95% CI 30·6–34·7) patients.
DISCUSSION
We demonstrated the impact of magnitude of bacteraemia on mortality in a large cohort of adult patients with first-time mono-microbial bacteraemia. Bacterial growth in all bottles of a three-bottle BC set predicted higher 30-day mortality and a 1-year excess mortality of 4·2%. This prognostic impact changed little when adjusted for a number of prognostically important covariates.
Most studies on magnitude of bacteraemia as a predictor have used direct measurements of c.f.u./ml blood [Reference Tilghman and Finland3–Reference Kreger9]. These methods are, however, labour-intensive and of low sensitivity due to small specimen volumes (1 ml); hence they are rarely used for routine diagnostic purposes [Reference Washington and Ilstrup23, Reference Yagupsky and Nolte24]. Our results are in agreement with these bacteraemia studies conducted in adults several decades ago [Reference Tilghman and Finland3–Reference Schønheyder11] (Table 2). However, none of the previous studies used multivariate analyses to control for potential confounding factors, only in-hospital mortality was reported, and the number of patients was below that of our study (ranging from 20 to 582).
Table 2. Prognostic studies that include magnitude of bacteraemia, adult patients
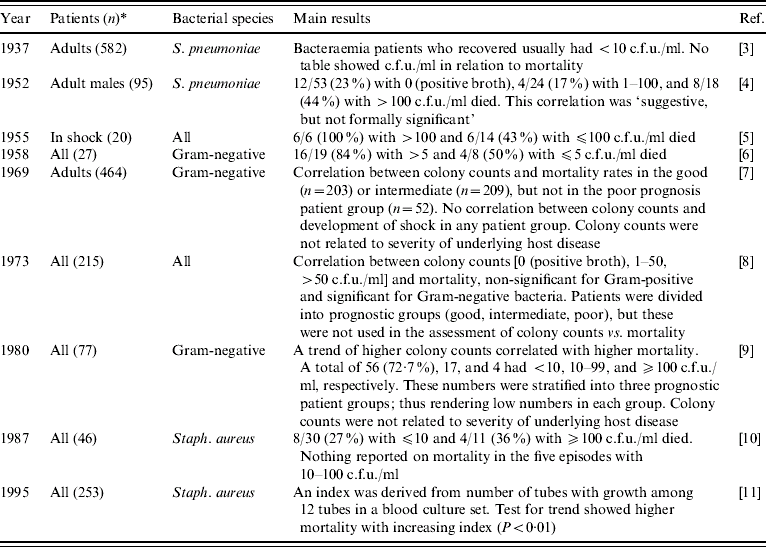
* Numbers may differ from those mentioned in the manuscript titles, as only bacteraemia specimens from adults in whom magnitude of bacteraemia was assessed are included in the table.
Recent studies reported that shorter time-to-positivity was associated with a worse prognosis in adult bacteraemia patients with Staph. aureus [Reference Khatib25, Reference Marra26], S. pneumoniae [Reference Peralta27], and E. coli [Reference Martinez28, Reference Peralta29]. Interestingly, Peralta and colleagues found an inverse relationship between time-to-positivity and numbers of positive BC bottles [Reference Peralta27, Reference Peralta29], indicating that both were indices of magnitude of bacteraemia and predictors of mortality. Unfortunately, we were unable to evaluate such a relationship in our study as only the time from sampling until notification was recorded consistently.
We find that the difference between BCI 1 and BCI 2 on the one hand and BCI 3 on the other seems plausible, as the former probably represent a few c.f.u./ml, whilst the latter represents a wider range from few to numerous c.f.u./ml. Still, the diverse results indicate that magnitude of bacteraemia exerts its effect differently in different patient groups.
A different impact of bacteraemia in males and females has been suggested in other studies [Reference Leibovici30]. The lack of association between higher BCI and mortality in the patient group without comorbidity underlines the importance of comorbid conditions. Further, the small impact of magnitude of bacteraemia in the oldest patient group suggests that these patients constitute a ‘survival cohort’ less sensitive to the detriments of infection. In medical patients the higher mortality was seen closer to the bacteraemic episode than in surgical patients, indicating that probably to a greater extent, infections form part of the primary disease entity in medical patients than in surgical patients.
For Gram-negative bacteria, DuPont & Spink found that mortality increased in parallel with higher c.f.u./ml [Reference DuPont and Spink7]. A successive study, including all bacteraemia patients, reported the same association for Gram-negative bacteria whereas the association was less clear for Gram-positive bacteria [Reference Kluge and DuPont8]; this is in accordance with our findings.
The main strengths of our study were the unselected cohort from a well-defined population, the high statistical precision, and complete follow-up. The Danish registries enabled 1-year follow-up beyond hospital discharge, which has rarely been reported [Reference Leibovici31]. Other strengths were the completeness of the bacteraemia data and almost 100% accurate registration in the Danish Civil Registration System [Reference Pedersen13].
Our study also had a number of limitations. First, we had no data on antibiotic consumption prior to the bacteraemic episode. Previous antibiotic treatment may explain the lower impact of magnitude of bacteraemia in nosocomial compared to community-acquired bacteraemias, as the former are probably more likely to have received antibiotics before venepuncture. Antibiotic treatment may result in negative BC results in some bacteraemia patients, probably those with the lowest magnitude of bacteraemia, but as early antibiotic treatment is also associated with a better prognosis, this would be in line with our findings. Second, we had no information on severity of the bacteraemic episode (sepsis, severe sepsis, and septic shock). However, the continuum from sepsis to septic shock is intermediate in the pathway from infection to death [Reference Martinez28, Reference Rangel-Frausto32] and should therefore not be considered a confounder [Reference Rothman33]. Third, we are aware that our BC practice with a blood draw of 30 ml distributed equally into two aerobic bottles and one anaerobic bottle differs from standard practice in most hospitals, which renders it difficult to replicate our study directly in other settings. However, the simultaneous draw of a high-volume sample has been recommended by others [Reference Li, Plorde and Carlson34, Reference Arendrup, Jensen and Justesen35] and it is unlikely that the clear associations between magnitude of bacteraemia and mortality and the biological plausibility of these findings have any relation to the use of a specific BC practice. Fourth, we are aware that the many subgroup analyses increased the likelihood of detecting accidental associations which were not necessarily biologically plausible, however, most results in subgroups were consistent with the overall results and the novel aspects also deserve attention in specific patient groups. Still, it remains to be explored whether these associations are causal or a marker of an underlying disease process unaccounted for by other known predictors.
In conclusion, magnitude of bacteraemia in adults, as indicated by a semi-quantitative bottle index, predicted higher mortality independently of other known predictors up to 1 year after diagnosis. Thus, the impact of magnitude of bacteraemia warrants consideration in future bacteraemia studies.
ACKNOWLEDGEMENTS
Financial support was received from the Department for University Relations, Aalborg Hospital, Aarhus University Hospital.
DECLARATION OF INTEREST
None.







