Introduction
Pneumonia is a common lung inflammation caused by bacterial or viral infection with a various number of symptoms and complications. It is associated with a high mortality, especially in the elderly population [Reference Wesemann1–Reference Raghavendran, Mylotte and Scannapieco3]. Also due to demographic trends towards a rapidly aging population, the number of elderly living in nursing homes (NHs) is expected to increase substantially in the future [Reference Richards4]. Alongside this development, the interest in research on pneumonia in this age group has raised rapidly during the last decades [Reference Ewig5]. NH residents suffering from any severe infection have an increased risk for hospital admissions and for a reduction in the general health condition [Reference Koch6]. Notably, NH acquired pneumonia mortality rates are higher than those caused by community-acquired pneumonia (CAP, 42% vs. 18% [Reference Liapikou7]). Older age (>70 years), male sex, alcoholism, tobacco smoking, swallowing difficulty and several chronic diseases (e.g. respiratory and cardiovascular diseases) have been identified as the most significant risk factors [Reference Raghavendran, Mylotte and Scannapieco3, Reference El-Solh, Niederman and Drinka8–Reference Loeb11]. Additionally, there is increasing evidence on the importance of the NH residents’ functional status (including physical, cognitive and social functioning). Studies from Asia [Reference Ma, Wah and Woo12–Reference Ugajin14] and the USA [Reference Muder15–Reference Mody, Sun and Bradley17] identified a poor functional status as a risk factor for pneumonia in long-term care facilities. To our knowledge no conclusive evidence from Europe is available.
In Europe, the annual incidence of CAP in the overall population ranges between 0.15% and 1.16% [Reference Almirall18, Reference Jokinen19], depending on the country and methods used. In Germany, the incidence of hospitalised CAP was 0.30% in 2006, higher for males (0.32% vs. 0.25%) and strongly age-related [Reference Ewig20]. A British study based on the Clinical Practice Research Datalink (CPRD) analysed hospitalised CAP in patients aged ⩾65 years between April 1997 and March 2011 and found an overall incidence of 7.99 per 1000 person-years (PY), which was higher in men than women. 10% of the CAP patients had no antibiotic treatment recorded on their date of diagnosis or the subsequent 28 days [Reference Millett21]. Vila-Corcoles et al. [Reference Vila-Corcoles22] included between 2002 and 2005 11 241 community-dwelling individuals aged 65 years or more in their study and estimated an all-cause CAP incidence (hospitalised and outpatient) of 14 cases per 1000 PY [Reference Vila-Corcoles22]. In a cohort of more than 9000 non-institutionalised persons aged 50–75 years in Germany, a cumulative 10-year-incidence of 4.5% was estimated [Reference Breitling23]. To date, there have been only a few population-based studies dealing with the incidence of pneumonia in NHs. A recently published Dutch study based on 341 NH residents’ electronic medical files from three NHs ascertained a cumulative incidence of NH acquired pneumonia of 9% per year [Reference Hollaar24]. The French incidence of pneumonia and related consequences in NH residents (INCUR) study analysed 773 elderly and recorded over a 1-year follow-up 160 (21%) incident cases [Reference Kelaiditi25]. Estimates of the incidence in German NHs are lacking. Considering this background, there is a need to amend the evidence by addition of information of the underlying factors that may be contributing to this disease. Therefore, this study aimed to calculate incidences of pneumonia in a large cohort of German NH residents and to compare the rates between sexes, age groups, care levels and comorbid conditions.
Methods
Data source and study design
We obtained data from the DAK-Gesundheit, a large statutory health insurance fund representing approximately 6 million members (corresponding to 7.5% of the German population [26]). Our cohort study included all those insured aged at least 65 years newly admitted to a NH between 1 January 2010 and 31 December 2014, preceded by a continuous insurance period of at least 365 days without a NH placement. Cohort exit was defined as the end of the study period (31 December 2014), the end of insurance, death, or the earliest diagnosis of pneumonia, whatever happened first. A re-entry after cohort exit was not possible.
In brief, the data contained information on demographics, the level of care dependency [Reference Busse and Blümel27], hospitalisations, outpatient care, as well as reimbursed outpatient drug dispensations. Hospital data hold information on the admission and discharge date, the respective diagnoses, as well as diagnostic and therapeutic procedures. Claims of outpatient care contain diagnoses including information on the level of diagnosis certainty (confirmed, suspected, ruled out and status post), treatments and procedures. All diagnoses are based on the German modification of the International Classification of Diseases, 10th Revision (ICD-10-GM). Outpatient drug dispensation data comprise information on all dispensations of reimbursable drugs and include amongst other things, prescribing and dispensation dates and the Anatomical Therapeutic Chemical (ATC) code.
Outcome and variables
NH residents were identified as pneumonia cases if they were diagnosed with a pneumonia ICD code as main discharge hospital diagnosis or confirmed outpatient diagnosis after NH placement. We adapted the respective ICD-10 code list published by the German Competence Network for Community Acquired Pneumonia (CAPNETZ [Reference Schnoor28], see Supplement 1). In Germany, an outpatient diagnosis can only be related to a calendar quarter. Therefore, the date of the outpatient diagnosis of pneumonia was defined as the start of outpatient treatment during the respective quarter. For inpatient diagnoses, we used the date of the respective hospital admission. We categorised a resident as inpatient or outpatient case according to his or her earliest diagnosis date after institutionalisation. Thus, a person was an outpatient case even if there was an inpatient diagnosis soon afterwards the outpatient one. Splitting the year in groupings of 3 months according to the meteorological seasons (December–February, March–May, June–August and September–November) we looked at the seasonal variation of the pneumonia occurrence.
The basic variables we assessed were age in four groups (65–74, 75–84, 85–94 and 95+years), sex and level of care dependency at NH placement as a proxy for the functional status of each resident. According to the German long-term care insurance people were classified to the levels of care 1, 2, or 3 due to the time required for daily help (1.5, 3, or 5 h, respectively). Care level 0 identifies persons with a minor need of care due to significant disabilities affecting their everyday life [Reference Schwinger and Jacobs29]. Because of the small number of residents with care level 0, the levels 0 and 1 were combined in this study. Comorbidities were assessed based on main discharge diagnoses, secondary hospital diagnoses or confirmed outpatient diagnoses in the 12-month baseline period preceding cohort entry. We considered the 31 disorders defined in the Elixhauser comorbidity index, we used the respective ICD-10-GM code list as published by Quan et al. [Reference Quan30] and we defined four risk categories (0–1, 2–4, 5–7 and 8+ comorbid conditions).
For incident pneumonia cases with an outpatient diagnosis, the proportion of patients receiving at least one antibiotic drug (ATC code J01) was described overall and stratified by an antibiotic agent. Since we are not able to see the exact date of an outpatient diagnosis, we considered drug prescriptions in the quarter of the diagnosis. For residents with an inpatient diagnosis, we analysed additionally the length of the respective hospital stay.
Statistical analysis
PY as the estimate of the actual time at risk in years that all NH residents contributed to the study were accumulated between the date of institutionalisation and cohort exit. The incidence of pneumonia per 100 PY was estimated by dividing the number of patients with pneumonia diagnosis by the total number of PY under risk. 95% confidence intervals (CIs) were calculated using the Poisson distribution [Reference Daly31]. All estimates were calculated for the overall study population and stratified by age, sex, care levels and comorbid conditions. Besides, the observation period was divided into 1-month intervals for the first year in the cohort, plus into five 1-year intervals. For the calculation of hazard ratios (HRs) with 95% CIs we used a multivariable Cox proportional hazard regression model, in which we determined factors potentially associated with pneumonia. Sex, age (in four categories), level of care dependency (in three categories), pneumonia before NH placement and all comorbidities (in four categories) were included as independent variables. We performed all statistical analyses with SAS for Windows version 9.4 (SAS Institute Inc, Cary, North Carolina).
Results
Baseline characteristics
The overall cohort included 127 227 persons (75% females) that were newly admitted to a NH between 2010 and 2014. Mean age at cohort entry was 82 years for males and 85 years for females. More than half the residents had the care level 0/1 (58.0%) at NH placement (see Table 1).
Table 1. Baseline characteristics of the study cohort and residents with pneumonia
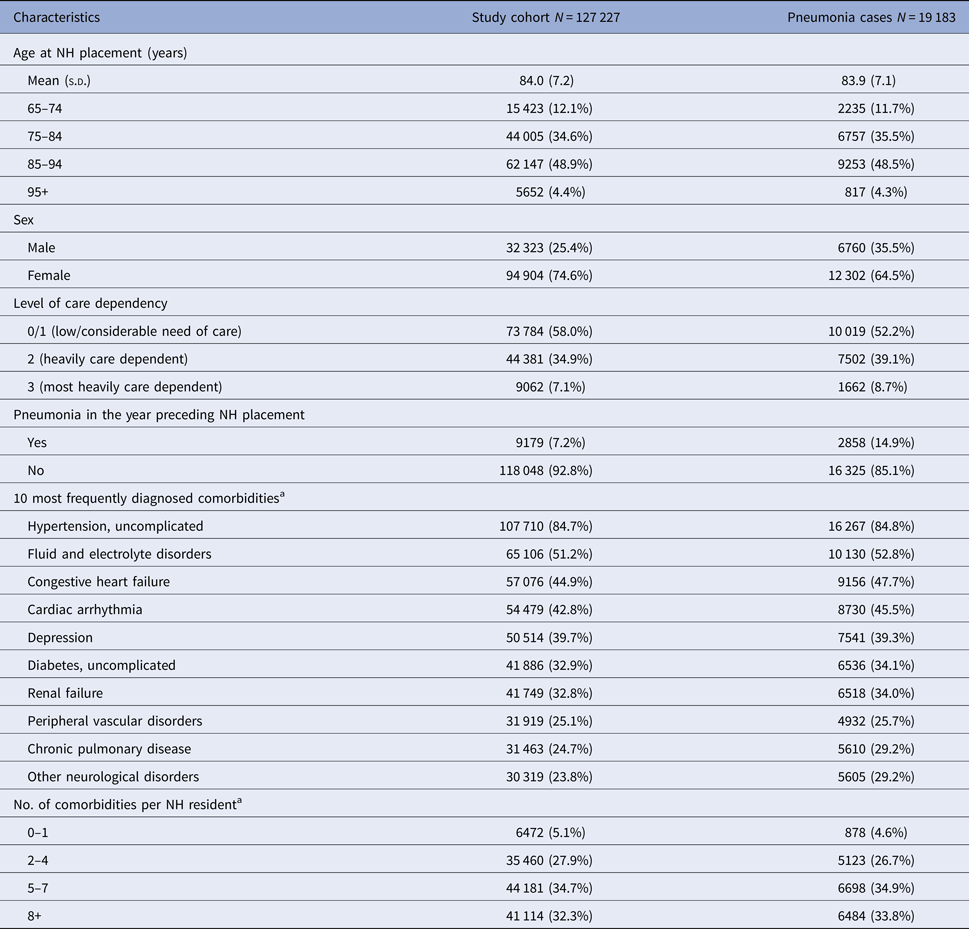
NH, nursing home; s.d., standard deviation; IQR, interquartile range.
a Assessed in the 12 months before cohort entry/NH placement.
A history of pneumonia in the year preceding NH placement could be observed in 7.2% of the overall cohort. The most common comorbidities were uncomplicated hypertension (84.7%), fluid and electrolyte disorders (51.2%), congestive heart failure (44.9%) and cardiac arrhythmia (42.8%). Most of the residents (34.7%) had diagnoses of 5–7 conditions (see Table 1).
Incidence
Over 5 years after institutionalisation, all NH residents accumulated 162 063 PY of follow up (median (IQR): 318 (82–732) days) and generated 19 183 cases of pneumonia which resulted in an incidence rate of 11.8 events per 100 PY (95% CI 11.7–12.0). The incidence in males was more than twice as high as in women (20.9 vs. 9.6). In the overall cohort, only slight differences between the four age groups could have been observed (see Table 2). The differences between age groups were more pronounced in males. While those 65–74 years old had an incidence rate of 17.4 per 100 PY, the oldest ones (95+) had 23.3 events per 100 PY. Furthermore, we could detect differences between the levels of care dependency of the residents. Persons with the lowest level 0/1 showed an incidence of 9.2 events per 100 PY, whereas the ones with level 3 (most heavily care dependent) had an almost three times as high rate of 25.1 per 100 PY. A history of pneumonia led to an incidence of 38.0 per 100 PY. Regarding the number of diagnosed comorbid condition, we could observe a twice as high incidence rate for NH residents with eight or more comorbidities compared with persons with one condition at most (see Table 2; for the single comorbidities: see Supplement 2).
Table 2. Incidence of pneumonia by demographic characteristics and selected comorbidities
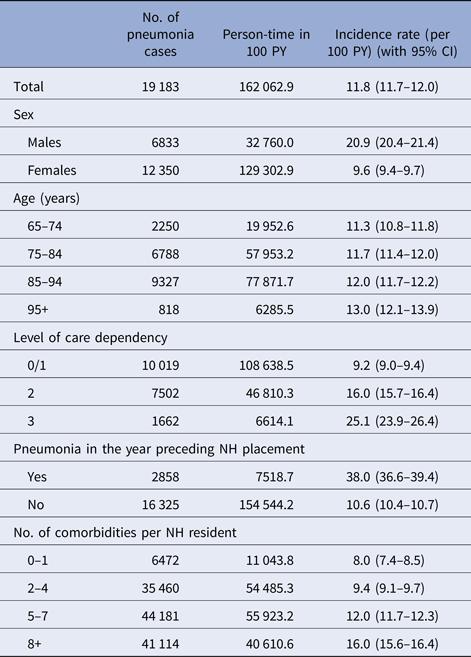
NH, nursing home; PY, person-years.
The results of the multivariate Cox proportional hazard model showed that among all included variables pneumonia in the year preceding NH placement was associated with the highest risk of a new episode after institutionalisation (HR 2.72, 95% CI 2.61–2.83). Further factors with a strong association were care dependency level 3 (compared with level 0/1; HR 2.14, 2.03–2.26) and male sex (compared with female sex; HR 1.88, 1.83–1.94). For all factors, the association was statistically significant (see Fig. 1).

Fig. 1. Cox proportional hazard ratios (HRs) for factors associated with pneumonia.
In the first year after institutionalisation 12 280 NH residents had pneumonia during 82 205 PY (14.9 per 100 PY, 95% CI 14.7–15.2). The incidence rate in males was almost 120% higher than in females. Figure 2 displays, that incidences were highest during the first month after NH placement (overall 34.4 per 100 PY, 95% CI 33.2–35.6), fell directly thereafter to 17.9 and reached a constant level of 10–11 per 100 PY from the fifth month onwards (see Fig. 2). The differences between sexes remained on a similar level, independent by time interval since NH placement.
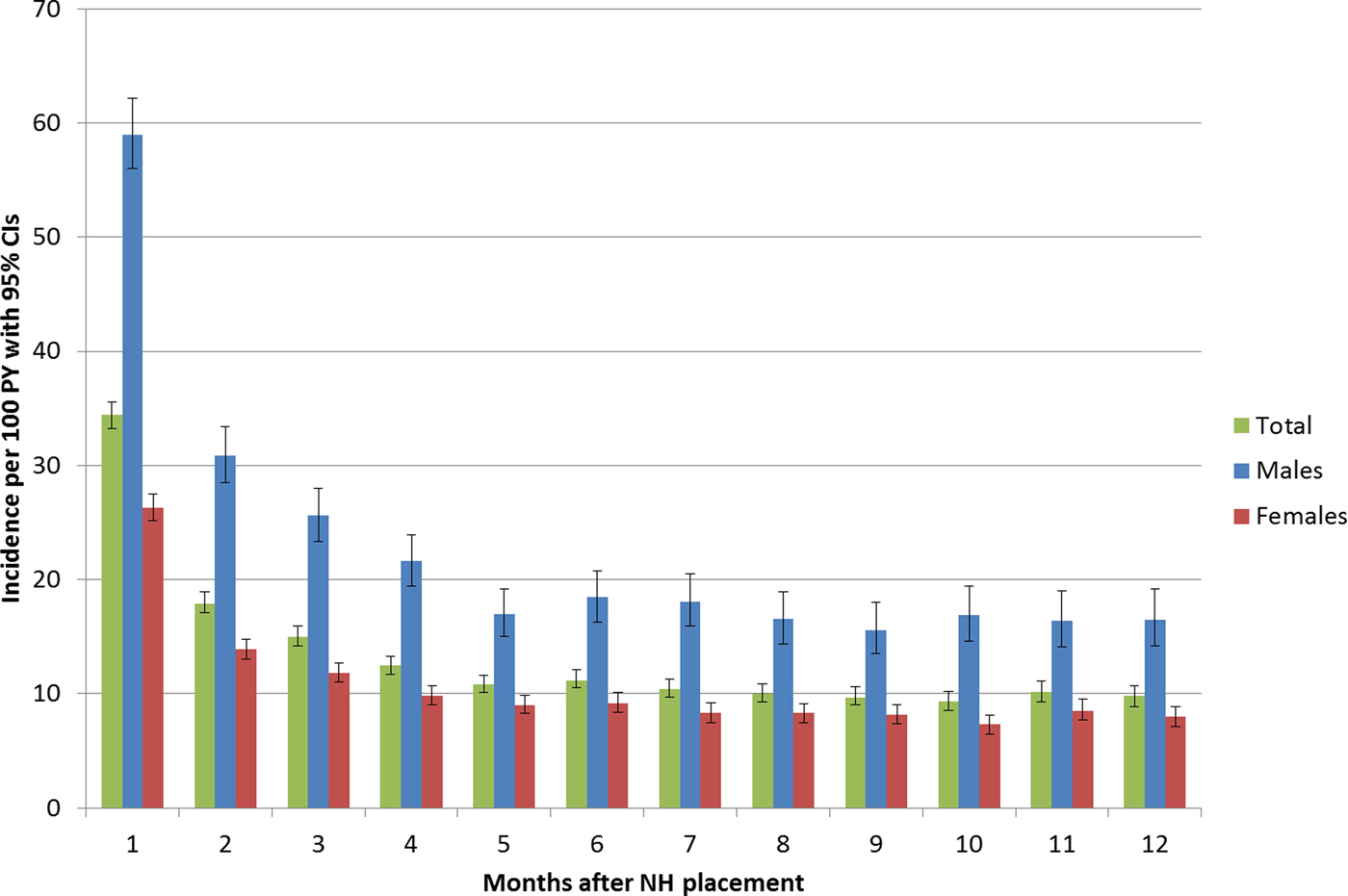
Fig. 2. Incidences per 100 person-years (with 95% confidence intervals) in the first 12 months after nursing home placement.
Treatment and outcome
Most of the 19 183 pneumonia cases occurred during winter (28.3%), followed by autumn (26.0%), spring (24.6%) and summer (21.2%). The distribution of demographic characteristics was similar as in the overall cohort with the exception of sex. Furthermore, in pneumonia cases, the proportions of comorbidities were slightly higher in most of them (see Table 1). Of all NH residents with pneumonia, 10 652 (55.5%) had an outpatient and 8531 (44.5%) had an inpatient diagnosis. 6958 outpatient cases (65.3%) had at least one antibiotic prescription during the quarter of their diagnosis, among them, 87.2% had one or two prescriptions. The most frequently prescribed agents were cefuroxime (16.1%), ciprofloxacin (11.8%), levofloxacin (8.9%), and amoxicillin (8.8%).
The residents with a hospitalisation due to pneumonia stayed on average 10.6 days in the hospital. In total 2391 (28.0%) persons died during their hospital stay.
Discussion
Pneumonia is one of the major causes of mortality in older adults [Reference Wesemann1] and because of its clinical relevance especially in NH residents this disease is a topic of particular interest [Reference Ewig5]. This study with over 127 000 newly admitted NH residents is to our knowledge one of the largest works examining the incidence of pneumonia in this population subgroup. Of all persons in our cohort, 15% (19 183) had a pneumonia diagnosis after their institutionalisation. The demographic characteristics and comorbid conditions were mostly comparable between residents with and without pneumonia.
The overall cumulative incidence in our cohort of 15% during the observation period of 5 years is right in the middle of the findings of Hollaar et al. (9% [Reference Hollaar24]) and Kelaiditi et al. (21% [Reference Kelaiditi25]). However, these studies are based on comparably small study populations with a follow-up of 1 year and incidence rates per PY were not calculated. According to the assumption that the incidence of NH acquired pneumonia is estimated to be six to ten times higher than the incidence of CAP [Reference Marrie32], our findings are much higher than the results reported by studies which analysed elderly living in the community [Reference Millett21–Reference Breitling23]. In general, differences in the results might be partly explained by variances in the applied methods (definition of pneumonia, incidence over time, study design, number of facilities evaluated, specific health care system).
Pneumonia in the elderly population is often treated in hospital. In a Spanish study of only community-dwelling individuals aged 65 years or more this led to a hospitalisation rate of 75% of CAP episodes [Reference Vila-Corcoles22]. We had in our institutionalised study population predominantly outpatient cases (55.5%). Hereto, it has to be considered, that we categorise a resident as an outpatient case even if there was an inpatient diagnosis soon afterwards the outpatient one. So it is mentionable, that 1801 outpatient cases (16.9%) had an inpatient diagnosis of pneumonia during the quarter of their outpatient diagnosis. Due to the uncertainty of the exact date of an outpatient diagnosis, we cannot exclude that some of these inpatient diagnoses were made before the outpatient ones.
Other factors seemed to increase the incidence of pneumonia which is in line with several other studies analysing the epidemiology of this disease in elderly in general and NH residents, respectively. Similar to Vila-Corcoles et al. [Reference Vila-Corcoles22], the incidence rates were twice as high in men than in women. Additionally, our HR for male sex (1.88) is quite similar to the finding of the multivariable analysis from Loeb et al. [Reference Loeb11]. Although this phenomenon is commonly shown, there is no conclusive evidence yet on the reasons for these differences between sexes. From the literature, it is known that males have higher hospitalisation rates in general (1.3-fold higher than in females after NH entry [Reference Hoffmann and Allers33]), for what the reasons are also still unclear. Further research should focus this issue and in general, we see that sex-stratified analyses in this population subgroup are essential. Slightly different from the evidence we found only minor increased estimates in older residents than in younger elderly (65–74 years). A pneumonia episode in the year before NH placement increased the incidence of a subsequent episode after institutionalisation [Reference Almirall18]. Similar as in the existing literature [Reference Raghavendran, Mylotte and Scannapieco3, Reference El-Solh, Niederman and Drinka8, Reference Torres10] we could ascertain increased incidences in residents with other single comorbid conditions, such as congestive heart failure or chronic pulmonary disease and in the same way in residents with multiple comorbidities. Referring to the association between the functional status of NH residents and the occurrence of pneumonia [Reference Ma, Wah and Woo12–Reference Mody, Sun and Bradley17], we could identify an almost three times higher incidence in residents with care level 3 (most heavily care dependent) compared with the ones who do only have a low/considerable need of care. Besides, a functional dependency is associated with a longer hospital stay, a higher mortality and a further functional decline [Reference Mody, Sun and Bradley17].
According to the current German guideline recommendations for the antibiotic treatment of patients with CAP or NH acquired pneumonia, respectively, differ by degree of severity. For patients with a mild or moderate pneumonia treatment is to be started with an aminopenicillin (e.g. amoxicillin) co-formulated with a beta-lactamase inhibitor or a second- or third-generation cephalosporin (e.g. cefuroxime). Alternatively, a fluoroquinolone (e.g. ciprofloxacin and levofloxacin) can be considered [Reference Ewig34]. A US American cohort study among NH residents with advanced dementia showed quinolones and third generation cephalosporins as most commonly used antibiotics [Reference D'Agata and Mitchell35]. However, the frequency and type of antibiotic use among NH residents vary within a healthcare system [Reference Daneman36] and between individual countries substantially [Reference van der Steen37].
The high proportion of outpatient pneumonia cases without a prescription of an antibiotic during the quarter of their diagnosis was unexpected, since according to the German guideline for the treatment of adult patients with CAP an antibiotic treatment is mandatory [Reference Ewig34]. Hereto, a look at the underlying ICD-10-GM codes is interesting. Two codes without an aetiological diagnosis represent 70.0% of all 3694 outpatient cases without an antibiotic prescription, namely J18.9 (pneumonia, organism unspecified) and J18.0 (bronchopneumonia, organism unspecified). This is not surprising since aetiological procedures are uncommon in the outpatient sector [Reference Vila-Corcoles22]. Residents with these ICD codes could partly suffer from viral pneumonia. The third most common code in this group was J69.0 (pneumonitis due to food and vomit). A diagnosis of pneumonitis, an inflammatory syndrome which does not typically require a therapy with antibiotics [Reference Raghavendran, Mylotte and Scannapieco3, Reference Mylotte38], should be considered in many NH residents with pneumonia [Reference Mills39]. Further possible reasons for this observation are hospital admissions soon afterwards the outpatient one (see above), persons who die during the quarter of their outpatient diagnosis (31% of 3694 outpatient cases) or an incorrect encoding, i.e. that one pneumonia ICD code is used although the respective NH resident suffers from another (respiratory) disease.
Limitations and strengths
Limitations are mainly attributable to the nature of the administrative data. The data were not captured for the purpose of scientific research and further health-related information that could influence the occurrence of pneumonia and the diagnostics are not available. Some misclassification of the outcome pneumonia is possible since for the identification of cases only diagnoses and their respective ICD codes were used and that they were not proved radiographically or validated by checking clinical records (similar applies to the comorbidities). Especially in the outpatient sector diagnoses are often unspecific because they are almost entirely made without further diagnostic procedures. This becomes apparent considering the two most frequently pneumonia ICD codes in the cohort, J18.9 and J18.0, both ones without further specification. Facing the hospital diagnoses, we expect a high validity because in Germany the coding of inpatient diagnoses is closely related to the payment in the form of diagnosis-related groups. Since the German claims data do not contain the exact date of an outpatient diagnosis, we used the start of the outpatient treatment as a proxy. The same applies to further information related to the specific resident's institution. These were not available for what reason other possible factors affecting the incidence, e.g. the NH's ownership or the resident's immunisation status, could not be investigated. Similar to other studies calculating incidences of pneumonia [Reference Breitling23, Reference Hollaar24], we used only the first pneumonia diagnosis after NH placement. Otherwise, we could not rate multiple diagnoses in one quarter as one or more pneumonia episodes. The data for this study were only obtained from one large health insurance fund and since the DAK-Gesundheit insures more females and a population with a generally higher burden of chronic diseases [Reference Hoffmann and Icks40], the results cannot be straightly extrapolated to the entire institutionalised population in Germany.
Strengths of the study are its real-world character, its large sample size which gave us the opportunity to stratify the incidences by sex, age and other variables and lack of non-response due to the administrative nature of the data. For every resident, information was available in the year before NH placement and up to 5 years thereafter. Furthermore, pneumonia treated in hospital are often in the focus of research [Reference Ewig5], whereas we also included cases diagnosed and treated outpatient.
Taken all into consideration, pneumonia is an important cause of illness and mortality in the institutionalised elderly population in Germany. More information about the epidemiology in this setting can only be generated by large population-based studies. Our results show that the summarised incidence of pneumonia in NH residents is relatively high and we could observe considerable differences between sexes and the care dependencies. In the upcoming years, further studies will be needed for the reasons of the substantial sex differences, for the detailed analysis of the antibiotic treatment and to assess possible preventive interventions in the health care system for an optimised care of this vulnerable population subgroup.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0950268818000997.
Acknowledgements
We thank the DAK-Gesundheit for contributing the underlying data for this analysis.
Declaration of interest
The authors declare that they have no competing interests.
Funding
There was no funding for this study.









