INTRODUCTION
Human listeriosis is caused by Listeria monocytogenes (Lm), a ubiquitous Gram-positive bacterial pathogen able to contaminate foodstuffs at any point in the food chain. From there, Lm is transmitted to humans through the consumption of a wide range of foods, but mainly through those classified as ready-to-eat [Reference Lianou and Sofos1]. Lm can produce severe invasive forms including septicaemia and, less frequently, central nervous system involvement in highest risk population as well as immunocompromised patients. Listeriosis also affects pregnant women and, although it can present as asymptomatic or weak (influenza-like) infections in the mothers, it may compromise gestation and cause abortion, premature birth, foetal death or severe neonatal disease. In immunocompetent people, listeriosis results in gastroenteritis, fever and the presence of asymptomatic carriers is also relatively frequent (5–10%) [Reference Allerberger and Wagner2]. Despite the low incidence of listeriosis among industrialised countries (0·3–0·7 cases per 100 000 inhabitants), the severity of the ill (fatality rate ≈20–30%) motivates the inclusion of this disease into the surveillance systems in developed countries [Reference Hedberg3, Reference Todd and Notermans4]. Surveillance systems, mostly passive and based on the notification of listeriosis cases, were developed in the 1990's after evidencing that Lm was transmitted to humans through contaminated food [Reference Schlech5]. Generally, for infectious diseases, and particularly for foodborne diseases, surveillance systems are only able to report a small fraction of the total cases, consequently underestimating their real incidence. Indeed, the process from exposure to a contagious agent, through consumption of contaminated food to effective detection of the case requires an occurrence of consecutive events, known as a ‘surveillance pyramid’ [Reference Haagsma6, Reference Gibbons7]. Therefore, either due to aspects of underdiagnosis or under-reporting, surveillance systems are able to report just the ‘tip of the iceberg’ [Reference Leclercq8]. Clearly, regarding more severe pathologies such as invasive listeriosis, which frequently entails hospitalisation of the patients and an unambiguous diagnosis, the underestimation should be lower.
Today, notification systems can be supplemented by molecular subtyping and supported by laboratory networks that allow to overcome the difficulties associated with conventional epidemiological investigations. In this direction the US surveillance of listeriosis was enhanced in 2004 by launching the programme ‘Listeria Initiative’ [9] as well as recently, the European Centre for Disease Prevention and Control (ECDC) launched a new version of the EPIS-FWD (Epidemic Intelligence Information System for Food- and Waterborne Diseases), which includes a new area named MTCI (Molecular Typing Cluster Investigations) [Reference Gossner10].
In Italy, notification of invasive listeriosis has been mandatory since 1990 and the available data have shown a non-homogeneous distribution of cases. In Lombardy, the national mandatory notification system (MAINF) has been computerised since 2005 and integrated with a Laboratory-based Surveillance System (LabSS), which allows for the collection and typing of the isolates from clinical cases at the Regional Reference Centre of the University of Milan.
Therefore, the aim of this study was to estimate the real incidence of listeriosis during a 9-year period (2006–2014), taking into account the availability of the two data sources in Lombardy Region, Italy.
METHODS
Characteristics of the Lombardy Region
The Lombardy Region (one of the 20 Italian regions) is located at the northwest of the peninsula. It is divided into 12 provinces and the regional capital is Milano. The Lombardy is the most populous region, accounting one-sixth of the Italian population (about 10 million people; 2% of the European Union population). The Lombardy is also the second most densely populated region in Italy with an elevated proportion of immigrants (10·5%), non-homogeneously distributed among the 12 provinces (from 13% in the provinces of Milano, Brescia and Mantova to 8% and 5% respectively in Como and Sondrio) [Reference Hook and Regal11].
The Lombardy Regional Health System is composed of 15 Local Public Health Units (LPHU), which almost coincide with the territory of the provinces. With regards to infectious diseases, clinicians are required to notify the corresponding LPHU on the basis of reasonable clinical suspicion. Once the diagnosis is confirmed by hospital laboratories, notification must be sent to the Regional Health Authority and then to the Italian Ministry of Health.
Data sources and case definition
In our study, all cases of listeriosis in people resident in the Lombardy Region and detected between 2006 and 2014 in at least one of two following data sources were included.
The MAINF
According to Italian law, clinicians are required to report each confirmed case of pregnancy and non-pregnancy-related listeriosis within 2 days to LPHU according to notification criteria, namely presence of compatible clinical symptoms and isolation of Lm from a normally sterile-site specimen. In Lombardy, these data are collected in a regional web-based system.
The LabSS
The LabSS started in 2005 through a joint initiative of the Lombardy Region and the Enterobacteria Regional Reference Laboratory of Department of Health Sciences University of Milan. Lm isolates from hospitalised clinical cases are collected by the LabSS and molecular typing (pulsed-field gel electrophoresis) of isolates is carried out in the Enterobacteria Regional Reference Laboratory. For the purposes of the present study, a listeriosis case was defined as one isolate of Lm from a normally sterile site (e.g. blood or positive cerebrospinal fluid) and a pregnancy-related listeriosis case was defined based on the isolation of Lm from a clinical sample of pregnant woman or foetus, stillborn, newborn aged <28 days. For pregnancy-related cases, each mother–infant pair was counted as a single case.
Capture–recapture (C–R) method
Listeriosis cases reported in Lombardy over 9 years (2006–2014) were cross-classified according to whether they were present or absent in each list (MAINF and LabSS). We used the C–R method to provide an estimation of the number of cases not captured by any data sources and consequently generate estimates of the real incidence of the disease [12, Reference de Sá13].
To use the C–R method, the following assumptions are necessary:
-
(1) Closed population, i.e. there is no change in the population during the investigation; we considered our population closed since migration bias is minimised by calculating the incidence for every year and excluding subjects not resident in the Lombardy region at the time of diagnosis;
-
(2) Unambiguous recognition and reliable matching of cases: record linkage for each case (name, birth date, sex and tax code) was applied to match data among the two data sources;
-
(3) No misdiagnosis: in our study the diagnosis for both sources (MAINF and LabSS) is confirmed by the isolation of Lm from a normally sterile-site specimen.
-
(4) Equal catchability: this is fulfilled when the probability of notification of one event is not influenced in each source by its characteristics (i.e. age, gender, severity of symptoms, circumstances of the diagnosis, etc.) and consequently every individual has the same probability of being reported;
-
(5) Independence of sources: the information flows of the two sources are clearly distinct and involve different healthcare professionals. Therefore, the event of one individual captured in one source is not dependant on its probability of notification in the other source. In our study the independence of sources was performed computing the OR as reported by Hook and Regal: ORs = 1 mean independence, ORs >1 indicate positive dependence and underestimation; ORs <1 indicate negative dependence between sources and overestimation [12–Reference Hook and Regal14].
RESULTS
Observed cases
Based on MAINF notifications and LabSS records, 580 cases of invasive listeriosis were observed in the Lombardy Region in the 9-year period considered. Of the 580 cases of invasive listeriosis, only 39 (6·7%) were pregnancy-related cases. Among non-pregnancy-related cases, 43·5% were females, 47·1% were younger than 70 years and 22·4% of the cases died. There were no meaningful differences between cases registered in both resources in terms of patients’ gender, age and survival.
From 2006 to 2011, the total number of observed cases as well as those reported only by MAINF and by only LabSS, increased from 43 to 93, from 35 to 75 and from 17 to 44, respectively. The observed distribution of cases, according to presence on each list by year and by Lombardy's province are presented in Table 1. As expected, data are unbalanced; the majority of patients (291 cases, 50·2%) were identified only via MAINF, whereas 97 (16·7%) were recorded only via LabSS; overlaps occurred between the two databases in 192 cases (33·1%).
Table 1. Listeriosis cases from two data sources (MAINF and LabSS) and total observed number (N) and incidence (per 100000 inhabitants) by years of diagnosis and Lombardy's provinces

* Population at 1st of January.
† Mean population between 2006 and 2014.
‡ The province is unknown for 11 patients.
The percentage of cases lost by MAINF, but detected by LabSS varies by year and province, from 8·9% in 2008 to 22·2% in 2014 and from 0% for the Province of Mantova to 42·9% for the Province of Lodi. The small number of all cases detected by the two data sources in the provinces of Mantova and Lodi did not allow for a reliable evaluation of the number of cases identified by the MAINF system because the observed differences of the percentage of cases detected only by LabSS (0% vs. 42·9%) could be random (P = 0·03).
The incidence of observed listeriosis cases (Table 1) varies from 0·46 per 100 000 inhabitants in 2006 to 0·96 per 100 000 inhabitants in 2011 from 0·22 per 100 000 inhabitants in the Province of Mantova and 1·11 per 100 000 inhabitants in the Province of Sondrio. Nevertheless, it seemed appropriate to apply the C–R method to get the best estimation of the incidence of listeriosis in the Lombardy Region.
Estimated cases
The C–R method assessment of the dependency of the two sources (MAINF and LabSS) was performed computing the OR 0·70 (95%, confidence interval (CI) 0·55–0·89); this result (OR significantly <1) indicates a slight overestimation in the real number of listeriosis cases. Nevertheless, the negative dependence observed overall, is not observed if the C–R estimation is computed stratifying by year of diagnosis or by Lombardy's provinces. Table 2 reports C–R estimates for listeriosis incidence by year of diagnosis, and province. The C–R estimation of listeriosis cases was 732 in the 9-year period (2006–2014), with an average of 20·8% of unobserved cases in total. The proportion of lost cases, computed as ‘(estimated-observed)/estimated’ from 2006 to 2014 (Fig. 1) show alternating years with a loss of cases below and above the average of 20·8%. In Fig. 2 the percentage of lost cases by province is reported. This value varies from 0% for the Province of Mantova to almost 50% for the Province of Sondrio.

Fig. 1. Percentage of lost cases computed as (estimated-observed)/estimated by years of diagnosis. The line is the mean value.

Fig. 2. Percentage of lost cases computed as (estimated-observed)/estimated by Lombardy provinces. The line is the mean value.
Table 2. Estimated (C–R method) number (N) and incidence (per 100 000 inhabitants) of listeriosis by years of diagnosis and by Lombardy's provinces
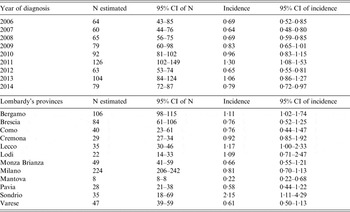
The lower limits of the 95% CI of estimated N and of 95% CI of estimated incidence are at least equal to the observed N and to the observed incidence.
We noted a progressive increase in the estimated incidence of listeriosis from 0·64 per 100 000 inhabitants (95% CI 0·48–0·80) in 2007 to 1·30 per 100 000 inhabitants (95% CI 1·08–1·53) in 2011, and a fluctuating incidence in 2012–2014. The estimated incidence varies by province: from 0·22 per 100 000 inhabitants (95% CI 0·22–0·68) for the Province of Mantova to 2·15 per 100 000 inhabitants (95% CI 1·11–4·29) for the Province of Sondrio. In particular, it is possible to identify three different areas according to the incidence: high (more than 1·00 per 100 000 inhabitants) in Sondrio, Lecco, Bergamo, Lodi; medium (0·60–1·00 per 100 000 inhabitants) in Cremona, Milano, Brescia, Como, Monza Brianza, Varese; and low (<0·60 per 100 000 inhabitants) in the remaining provinces (Fig. 3).

Fig. 3. Geographical distribution of the mean annual estimated incidence (cases/100 000 inhabitants) of listeriosis per year in Lombardy Region during the period 2006–2014. Provinces: BG, Bergamo; BS, Brescia; CO, Como; CR, Cremona; LC, Lecco; LO, Lodi; MN, Mantova; MI, Milano; MB, Monza Brianza; PV, Pavia; SO, Sondrio; VA, Varese.
By the C–R method the estimated mean annual incidence of listeriosis was 0·84 per 100 000 inhabitants (95% CI 0·66–1·03). If the real incidence of listeriosis was equal to 1·03 per 100 000 inhabitants per year (upper limit of the 95% CI of the estimated mean annual incidence by the C–R method) in the Lombardy Region, up to 35 additional cases of listeriosis per year than those identified by the two surveillance sources should be reported.
DISCUSSION
Although in Italy listeriosis has been included since the early 1990s in the Italian notification-based surveillance system, data on the burden of this atypical foodborne disease are still very limited. The aim of the current study is to illustrate how the Lombardy enhanced surveillance system, based on the MAINF and the LabSS, allows for the acquisition of more reliable estimates of listeriosis incidence.
In the considered period, the observed mean annual incidence of listeriosis was 0·67 per 100 000 inhabitants per year, the estimated (C–R method) incidence was 0·84 (95% CI 0·66–1·03). Both the observed incidence and the estimated incidence were the highest in 2011.
The increase of listeriosis cases in France and other European countries since 2006 [Reference Goulet15, Reference Denny and McLauchlin16], as well as that observed in this study in the Lombardy Region in the subsequent years (peaking in 2011), may be due to a real increase in the number of cases or to an improved surveillance systems sensitivity. Even in Italy there has been a gradual increase in the number of listeriosis cases reported, from <50 cases per years in the period 1996–2004 to over 100 per year in the period 2008–2013 (last year available). In the considered period, based on the notification system, in Italy the mean annual incidence (0·20 per 100 000 inhabitants) was lower than in the Lombardy Region (0·56 per 100 000 inhabitants) and in Europe (0·30 per 100 000 inhabitants in the period 2007–2014) [17–19]. In Lombardy, the incidence calculated based on the number of the observed cases is variable depending on the year and on the province. In particular, the highest mean annual incidence of listeriosis was found in 2011 (0·78 per 100 000 inhabitants when considering only the official notifications; 0·96 per 100 000 inhabitants when considering both sources). In the same year, only in Denmark the notification incidence (0·88 per 100 000 inhabitants) was higher than in the Lombardy Region [18].
The differences observed between countries and between areas within the same country may be attributed to three factors: (1) different eating habits, (2) a non-homogeneous distribution of risk factors for listeriosis, and lastly (3) different surveillance sensitivity that often is limited to traditional notification system. With regard to the first factor, is interesting to observe that the four provinces (Sondrio, Lecco, Bergamo and Lodi) with higher incidence match with those characterised by traditional production and consumption of soft cheeses (‘Taleggio’ and ‘Gongorzola’) made from raw milk. It could be interesting to confirm these observations through ad hoc studies.
As for the heterogeneous distribution of listeriosis risk factors, there was a higher frequency of conditions in the Lombardy population that can favour the increase of listeriosis cases [Reference Allerberger and Wagner2]. These conditions include older age (21% of the population is older than 65 years [Reference Hook and Regal11]), the prevalence of immune-suppressing diseases – such as cancer (prevalence = 3·4% [20]), but also the presence of socio-economic determinants, such as belonging to specific ethnic group (immigrants in Lombardy more than 1 000 000 [Reference Hook and Regal11]), and economic disadvantage. The aforementioned risk factors were observed in both England and Wales [Reference Mook21].
Finally, concerning the differing sensitivity of surveillance systems, the highest incidence calculated for the Lombardy Region is attributable to a greater diagnostic capacity and a more efficient detection system of cases. Certainly, our findings are similar to that reported by those countries where surveillance activity has been enhanced and supported by laboratory surveillance, as by Denmark since 1993 [Reference Gerner-Smidt22] and Netherlands since 2005 [Reference Doorduyn23].
In the considered 9-year period, only 83% of the observed cases (483 cases, 53·7 cases per year) were detected by MAINF. In 2011, when the peak incidence was observed with 93 total cases, the MAINF system detected only 75 listeriosis cases, with a loss of 19·4%, while LabSS detected the highest absolute number of cases (44). The reasons for the under-reporting are different for the two sources. Since MAINF system detects only telematics notifications about patients that, in most cases, are hospitalised, the lack of notification may be due to the communication and/or management issues into the health departments, even in the presence of a diagnostic assessment. Therefore, in many cases the onset of infection by Lm can be overshadowed by severity of the underlying disease (cancer, etc.). Regarding the LabSS system, the under-reporting may be due to logistical difficulties in sending the bacterial isolates, as might have happened to the laboratories in the provinces of Sondrio and Mantova, which are the furthest from the Regional Reference Centre, in Milano. Another reason may be the different sensitivity of the operators involved in the surveillance activities on a voluntary basis. In the last year of the study (2014), the training initiatives promoted at the regional level, the constant comparison with the notifications database (easily accessible by the reference laboratory) and the continuous and timely reminder sending isolates by laboratory network led to an increase of cases detected from both sources (52·8%), but not to a complete overlap of the collected information.
However, the incidence calculated on the basis of the cases observed by both information sources may be an underestimation of the real incidence. Therefore, for the estimated listeriosis cases that have occurred from 2006 to 2014, from the two information sources MAINF and LabSS, the C–R method was applied. To obtain more reliable estimates, in analogy to what has been achieved by several authors [Reference Gallay24, Reference Nardone25] the use of a potential third source, represented by hospital discharge records was evaluated. The information contained in these records is not independent from those contained in MAINF system, as it is required by the C–R method.
The estimated number of listeriosis cases (C–R method) in the period 2006–2014 is 732 cases (about 81 per year), corresponding to a mean annual incidence of listeriosis of 0·84 per 100 000 inhabitants. This value is significantly higher than that estimated for Western Europe (0·342, 95% CI 0·284–0·405) according to the meta-analysis of Maertens de Noordhout et al. [Reference Maertens de Noordhout26], but also with a presumed and not-inconsiderable loss in the number of cases of approximately 21% in total. This loss is equivalent to a correction factor for the under-reporting equal to 1·26, much lower than that calculated for Salmonella infections (correction factor = 17) and very far from that for Campylobacter infections (correction factor = 100) [Reference Flint27]. These data were expected, considering the seriousness of the disease, but requires to be examined in more detail, since it does not explain the differences observed by the years and the provinces. The likely lower sensitivity of the system to detect pregnancy-related cases must be taken into account. In fact, regarding pregnancy-related cases, in addition to the under-reporting, there may be an underestimate due to the underdiagnosis. In Lombardy Region, only the 6·7% of all observed cases is pregnancy related. This percentage is significantly lower than that reported in the meta-analysis (20·7%) promoted by World Health Organization [Reference Maertens de Noordhout26] and to that reported in France (18%) in a study lasting over 10 years (1999–2011) [Reference Girard28].This difference might be due to the fact that, while in the diagnostic assessment of non-pregnancy-related cases blood culture or CSF culture are routine procedures in the presence of septicaemia or central nervous system involvement, respectively, the diagnostic assessment in pregnancy-related cases is not consolidated, even when serious events occur, such as foetal death [Reference Siegman-Igra29].
The surveillance system in use in the Lombardy Region can still be improved, and the organisational model adopted could be extended to the other regions, with the support of the National Reference Centre (Istituto Superiore di Sanità, ISS) and the hospital laboratories, in order to overcome the current notifications system and gradually improve the surveillance of listeriosis. The surveillance system is aimed to promptly recognise foodborne outbreaks and carry out epidemiological investigations. Questionnaires represent an important tool to investigate and solve foodborne outbreaks in the USA, as reported by CDC (Centre for Disease Prevention and Control) [30]. However, the use of such questionnaires about food exposures in Italy (both at national and regional level) have some limitations, mainly due to the huge variety of food production, often characterised by a local distribution. Therefore, since it is very difficult to identify invasive listeriosis outbreaks using conventional epidemiology alone, molecular subtyping methods, and in particular Whole-Genome Sequencing, have proven to be an essential tool. Some initiatives have already been undertaken in this direction in recent times, and will help to better define the burden of listeriosis in Italy. In particular, the systematic sharing of the results of molecular typing of Lm strains, coordinated with all other European countries through the ECDC, could increase the ability of surveillance to identify outbreaks (often international) and reconstruct the infection chain [Reference Gossner10]. The joint use of data provided by notifications and by isolate subtyping will be able to overcome the difficulties in conducting epidemiological investigations and identifying outbreaks and to assess the effectiveness of interventions both for prevention and control carried out throughout the food chain and for the adoption of correct food behaviour. In fact, the variability in the incidence of the disease during the study period, in addition to underdiagnosis and under-reporting, can be influenced by the adoption of several measures to control the agro-food chains and the initiatives aimed to change eating habits of subjects at risk, as was the case in France, where there has been a particular focus on risk factors for listeriosis, or in the USA where the aim is ‘zero tolerance’ [Reference Girard28, Reference Tappero31].
ACKNOWLEDGEMENTS
The authors would like to thank M. Gramegna and A. Piatti at Directorate General of Welfare of Lombardy Region for allowing the access to the MAINF database. This research received no specific grant from any funding agency, commercial or not-for-profit sector.
DECLARATION OF INTEREST
None.










