Streptococcus agalactiae, also known as group B Streptococcus (GBS) is the leading cause of meningitis, pneumonia and bacterial sepsis in neonates in the USA and Europe [Reference Tettelin1]. Maternal intrapartum GBS colonization is a risk factor for early onset GBS disease and this has been linked with vertical transmission of GBS in over 95% of neonatal carriers or cases, the remainder being accounted for by rare cases of transmission through human milk and by nursery personnel [Reference Spellerberg2].
Studies aimed at developing a GBS vaccine are ongoing worldwide and attention has focused on the contribution to immunoprotection of the capsular polysaccharide antigen (CPS) and its potential as a vaccine target [Reference Johri3]. Nine serologically distinct capsular polysaccharide serotypes had been identified until relatively recently in GBS, namely Ia, Ib, II, III, IV, V, VI, VII, and VIII; a further serotype has latterly been added, designated serotype IX [Reference Slotved4]. It has been shown previously that predominating GBS serotypes change over time, vary with ethnic origin and can be associated with different diseases [Reference Johri3, Reference Campbell5]. Knowledge of local GBS serotype distribution is therefore important for the development of an effective vaccine tailored for a target population. To this end the current study assessed the prevalence and serotype distribution of GBS in a large population of women of child-bearing age in southern Ireland.
Two thousand (1000 each between October and November 2004, and between October and November 2006, respectively) non-duplicate vaginal swabs from females aged between 15 and 54 years at Cork University Hospital were screened for the presence of GBS. All swabs were anonymized and assigned a unique identifying number for the purpose of the study. Swabs were cultured on Islam agar (Oxoid®, UK) and incubated anaerobically at 37°C for 48 h. Orange-red colonies were subcultured on Columbia blood agar base (Oxoid) and incubated in 5% CO2 atmosphere at 37°C for 18–24 h. Presumptive GBS colonies were assigned to Lancefield groups using the Remel Streptex® kit (Launch Diagnostics, UK) according to the manufacturer's instructions. Isolates were stored on cryobeads at −70°C (Technical Service Consultants Ltd, UK).
A total of 323 GBS isolates were serotyped by latex agglutination with a serotyping kit (Essum AB, Sweden) consisting of nine antisera to serotypes Ia–VIII. Non-reactive isolates were further tested with the 10-serum kit (Ia–IX) [Statens Serum Institut (SSI), Denmark]. Isolates that failed to type by either serotyping kit were deemed serologically non-typable. All serotype IV isolates initially identified using the Essum Probiotics kit, except for three unrecoverable isolates, were re-tested using the SSI kit, as were a set of 15 isolates that included a variety of serotypes (but excluding serotype IV). All of these isolates gave concordant results for the two serotyping kits.
The genetic relatedness of serotype IV isolates was investigated using random amplified polymorphic DNA analysis (RAPD). Purified DNA was extracted from a 10 μl loopful of agar culture emulsified in 400 μl of phosphate buffered saline; 10 μl of 10 mg/ml lysozyme (Sigma Aldrich, Ireland) was added to this suspension and the solution was incubated at 37°C for 15 min, and DNA was extracted using the MagNA Pure Compact Nucleic Acid Isolation kit according to the manufacturer's instructions (Roche Diagnostics, Ireland). The concentration of DNA from each sample was quantified spectrophotometrically and adjusted to 100 ng/μl. RAPD fingerprinting was performed according to a previously published method [Reference Martinez6] (and using the primer OPB18), in duplicate to ensure reproducibility. A dendrogram of the relatedness of the RAPD profiles was constructed using the DendroUPGMA program (http://genomes.urv.cat/UPGMA).
The GBS colonization rate was 16·1% (323/2000) which is comparable with the rates reported from Western Europe (11–26%) [Reference Kieran7, Reference Barcaite8] but below rates from Eastern Europe (20–29%) and Scandinavia (24–36%) [Reference Barcaite8]. We employed a GBS selective agar as a screening tool because it has been suggested that along with enrichment broths this improves the sensitivity of GBS screening tests [Reference Bergeron and Ke9]. The addition of an enrichment broth might well have increased the detection rate further. The pregnancy status of women in this study was not established as it was previously shown that there is no difference in colonization rates between pregnant and non-pregnant women of child-bearing age [Reference Brimil10].
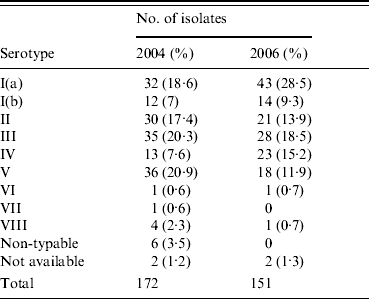
In the current study all serotypes except IX were detected with Ia, II, III, IV, and V predominating as shown in Table 1. The latter types in addition to serotype Ib accounted for 305/323 isolates (94·4%) with Ib being the least frequent. Serotype Ia increased in prevalence from 18·6% to 28·5% between the two sampling periods, while serotype V decreased from 20·9% to 11·9%. However, the most dramatic finding in this study lay in the prevalence of serotype IV, at 11·2% (7·6% of the 2004 isolates and 15·2% of the 2006 isolates) which contrasts with an earlier Dublin study where serotype IV prevalence was 1·9% [Reference Dore11]. The emergence of this serotype also contrasts with global epidemiological studies, where it had only been previously reported in significant numbers in colonizing and invasive GBS strains isolated in the United Arab Emirates (26·3%) and in other countries of which Turkey shows the highest prevalence at 8·3% [Reference Amin, Abdulrazzaq and Uduman12, Reference Ekin and Gurturk13]. Fewer than 2% of isolates in the current study were serologically non-typable.
Table 1. Serotype distribution in 323 GBS isolates collected in 2004 and 2006 from women of child-bearing age
RAPD analysis of 33/36 serotype IV isolates (data not shown) revealed seven distinct clusters which contained two or more isolates suggesting considerable genetic heterogeneity within the serotype.
Serotypes Ia, Ib, II, III, and V are associated with neonatal invasive disease [Reference Blumberg14]. Concurrently with this study GBS was isolated by our department in 13 cases of neonatal invasive disease with the following serotypes identified: Ia (n=4), Ib (n=2), II (n=1), III (n=6). It is noteworthy that serotype Ib, which accounted for 8·1% of colonizing strains, was represented by two of the 13 invasive isolates, while serotype V (16·7% of the colonizing strains) was not identified in the invasive isolates.
In conclusion, although the GBS colonization rates are relatively low at 16·1% in the current study, we have highlighted the unexpected emergence of serotype IV. This serotype may need to be included in any GBS vaccine developed for use in Ireland. To date, no vaccine incorporating serotype IV has reached clinical trial, but it has been included in at least one vaccine under consideration [Reference Paoletti and Kasper15].
ACKNOWLEDGEMENTS
The purchase of reagents for this project was funded jointly by the Department of Medical Microbiology, Cork University Hospital and by Cork Institute of Technology, under a seed funding scheme. Anonymized samples were provided by the Department of Medical Microbiology, and we thank our colleagues for their support of this project.
DECLARATION OF INTEREST
None.





