Sulfur mustard (SM) is a highly cytotoxic alkylating and blistering agent originally used during the First World War to inflict mass casualty and incapacitation among combatants. Reference Pechura and Rall1 Consequently, SM has historically been regarded as a chemical weapon of mass destruction (CWMD) threat only to the military. However, based on more recent state and nonstate-sponsored civilian mass casualty incidents in the Middle East, Reference Razavi, Razavi and Pirhosseinoo2–Reference Sezigen, Eyison and Ortatatli5 SM is considered by the United States Department of Homeland Security (DHS) as a chemical of concern (COC). 6 Compounds deemed by DHS as COC are extremely hazardous and are considered high-consequence public health threats whether released intentionally or accidentally and have the potential to cause both acute and long-term toxicities.
The primary modes of SM exposure are inhalation, ingestion, dermal, and ocular. Depending on the route and dose of exposure, the physical effects of injury may not be readily apparent until several to 24 h after initial exposure, which complicates the potential for early diagnosis and treatment. SM is best known for its vesicating properties, with characteristic large fluid-filled blisters at contacted areas of the skin Reference Ghabili, Agutter and Ghanei7 ; the inhalation and ocular toxicities of SM exposure have also been well-characterized. Reference Ghanei and Harandi8,Reference Panahi, Roshandel and Sadoughi9 While not as extensively studied, hematologic corollaries of SM exposure have long been reported to exist. Reference Needham, Cohen and Barrett10,Reference Hassan, Ebtekar and Ghanei11 Of important medical concern following severe SM exposure is the prospect of bone marrow suppression and the resulting manifestation of pancytopenia. Although rarely reported clinically, these hematological effects are considered the most serious complicating toxic effect of SM exposure. Reference Tabarestani, Balau-Mood and Farhoodi12 The reductions in critical immune cell populations are likely to increase susceptibility to infection, thereby introducing secondary morbidities that can further complicate patient treatment and prognosis.
Treatment with immunostimulant therapies represents a promising medical countermeasure (MCM) strategy to address the immunosuppressive effects of SM. Increasing neutrophil production with granulocyte colony-stimulating factors (G-CSF; filgrastim) Reference Frampton, Lee and Filgrastim13 can successfully treat neutropenia induced by exposure to anti-cancer drugs Reference Dale, Crawford and Klippel14 and acute radiation. Reference Farese, Cohen and Katz15,Reference Harrold, Gisleskog and Perez-Ruixo16 Similarly, a previous pilot study using a nonhuman primate model of SM exposure successfully demonstrated an immunostimulant effect following filgrastim treatment. Reference Anderson, Holmes and Lee17 More recently, in 2016 during the Syrian Civil War, G-CSF was administered off label to SM-exposed neutropenic patients which resulted in positive clinical outcomes. Reference Kilic, Ortatatli and Sezigen3 Together, these observations suggest that G-CSF is a promising MCM candidate that could be used to mitigate the myelosuppressive effects of SM exposure. However, as promising as these observations appear, further characterization of filgrastim as a candidate adjunct MCM in a well-controlled, scientifically rigorous study is still warranted.
Neupogen® was approved by the US FDA in 2015 for treatment of adult and pediatric patients acutely exposed to myelosuppressive doses of radiation (Hematopoietic Syndrome of Acute Radiation Syndrome, or H-ARS). 18,Reference Singh, Romaine and Newman19 Consequently, Neupogen® presents an exciting opportunity as a broadly acting MCM effective against both chemical and radiological threats.
As such, the purpose of the present study was to report data that further support the potential clinical utility of filgrastim, more specifically Neupogen®, as an MCM for SM exposure. The results reported here were derived using a recently developed small animal MCM efficacy screening platform that replicates many of the key toxic hematological aspects of SM exposure. Reference Beske, Wilhelm and Harvilchuck20 In this model, rats are challenged with SM through the intravenous (IV) route to specifically separate the myelosuppressive consequences of SM exposure from other potentially confounding injuries commonly arising from dermal, ocular, or inhalation exposures. With this approach, the model can identify MCMs that could be used as adjunctive treatments for systemic SM toxicity in combination with those that would be administered for tissue-specific injuries (ie, skin, eye, and lung). In this study, efficacy of the G-CSF drug Neupogen® as a potential MCM to mitigate SM-induced systemic toxicity was evaluated by means of clinical observations, body weights, body temperatures, complete blood count (CBC) profiles, and mortality rates relative to vehicle-treated controls. Treatment commenced 1 d after SM exposure, a time-point coinciding with evidence of early-onset lymphopenia and representing a realistic time-frame for postexposure treatment in many potential real-world settings.
Methods
Animals
For pharmacokinetic/pharmacodynamic (PK/PD) studies, male Sprague-Dawley rats with surgically implanted jugular vein catheters were obtained from Chares River Laboratories (Hollister, CA). The animals were 397-435 g and 14-15 wk of age at the time of the first Neupogen® dose. For SM studies, male Sprague-Dawley rats with surgically implanted jugular vein catheters were obtained from Charles River Laboratories (Raleigh, NC). The animals were 386-464 g and were 14-16 wk of age at the time of SM challenge. Animals were sedated by CO2/O2 and implanted with programmable temperature transponders (IPTT-300, Bio Medic Data System) posterior to the jugular vein catheter during quarantine. Animals were maintained on a 12-h light/dark cycle with no twilight. Air temperature in animal rooms was maintained within a 16 to 27°C range, with relative humidity maintained between 30 and 70%. Food and water were provided ad libitum. Only animals that were free of malformations and exhibited no outward signs of clinical diseases were placed on study. To ensure similar mean body weights across study groups, animals placed on study were randomized by weight.
SM Exposure
Distilled SM was acquired through the US Army Edgewood Chemical and Biological Center (Aberdeen Proving Ground, MD) as part of an Interagency Agreement between the National Institutes of Health (NIH) and the Department of Defense. Purity analysis, preparation of SM dosing solutions at 1.5 mg/mL in ethanol/propylene glycol, dose confirmation analysis, and intravenous (IV) tail vein dose administration were performed as previously described. Reference Beske, Wilhelm and Harvilchuck20
Neupogen® Treatment
Neupogen® (filgrastim), manufactured by Amgen® (Thousand Oaks, CA), was purchased by NIH and provided to Battelle by SRI International (Lot #1098089). Animals received Neupogen® treatment by the subcutaneous (SC) route as a single daily injection at 41 µg/kg/d (see the Results section for dosing justification). Final dosing solutions of Neupogen® were prepared by dilution in 5% dextrose (Hospira; Lot # 91-209-06) to a working concentration of 8.2 µg/mL. Dose formulations were prepared fresh daily at room temperature in glass vials. The formulations (dilutions) were mixed well by gentle inversion and stored at 4°C in a refrigerator and protected from direct light until use. Dose solutions were brought to room temperature before treatment. Dextrose (5%) was used as the treatment vehicle. Vehicle and Neupogen® treatments were performed at a dose volume of 5 mL/kg through SC injection at rotating sites on the mid-dorsum to avoid contact with catheter lines and temperature chip implants on the scapulae. Treatment was initiated 1 d after SM exposure and was continued daily for 8 d. For all treatment days, treatments were performed in the afternoon between 1300 and 1500 hours.
Clinical Observations
Twice daily clinical observations (morning and afternoon) for signs of toxicity were recorded during quarantine, before IV delivery of challenge material, and at all study days following challenge except the day of euthanasia where a single morning observation was collected.
Body Weights and Body Temperatures
Animals were weighed during quarantine (for randomization), on Day -1 (for dosing calculations), before challenge on Day 0 (for baseline), and daily post-challenge thereafter through the scheduled terminal time-point. To track body weight gain/loss, body weights were normalized to each animal’s individual baseline (Day 0) weight and expressed as a percentage of baseline. Body temperatures from the IPTT-300 were recorded daily in the morning starting on Day -3, on the day of challenge (Day 0) before administration of challenge material and continued daily thereafter through the scheduled terminal time-point.
Hematology
Whole blood samples were collected from the jugular vein catheter or directly from the jugular vein (if the catheter was not functioning) from unanesthetized animals. For hematology, blood was transferred into K3EDTA tubes and retained at room temperature until same-day hematology analysis on an Advia 120 Hematology Analyzer. A blood sample containing clots (1 of 210 total draws) was excluded from analysis and graphing.
PK/PD
Animals were dosed subcutaneously at either 30, 60, or 120 µg/kg of Neupogen® at a dose volume of 5 mL/kg. Blood was collected at the indicated time-points through the jugular vein catheter (or another accessible vessel). For drug level (PK) determination, blood was processed to plasma and analyzed using a bioanalytical method established at SRI using a commercially available human G-CSF enzyme-linked immunosorbent assay kit. For PD determination, a hematology analysis was performed on K3EDTA-treated whole blood using absolute neutrophil counts as a measure of the PD effect of Neupogen®. Analysis was performed using Phoenix® WinNonlin®.
Statistical Analyses
Statistical analysis was performed using SAS® (version 9.4) or R (version 3.6.0). For comparisons with more than 2 groups, analysis of variance (ANOVA) models fitted separately to temperature, body weight, and each hematology parameter with effects for group, day, and the interaction between group and day were used to assess the model assumption of normality. Standardized residuals from these ANOVA models were obtained, and a hypothesis test was performed for each parameter to assess the model assumption of normality for the untransformed data. Each parameter was then transformed by taking the base 10 logarithm of the parameter values. The ANOVA models were then refit using the base 10 log-transformed values, and a hypothesis test was again performed for each parameter to assess the model assumption of normality for the log-transformed data. If the assumption of normality was more reasonable for the log transformed data than it was for the untransformed data, then the log-transformed data were used throughout the analysis for this parameter. For hematology data plotted on a Log y-axis, data reported as 0 were reported as 0.001 (an order of magnitude below the lowest non-zero value on the analyzer) to allow for all collected data to be plotted. When appropriate, individual hematology cell count outliers (6 total samples out of 210 analyzed) were removed by a Grubb’s test (P < 0.05) for analysis and graphing. For survival analysis, the survival proportion and 95% Clopper Pearson confidence intervals were calculated for each group. Boschloo’s 2-sided tests were performed to determine if the proportions of surviving animals were significantly different between the SM-challenged groups only. Log rank tests were performed, and Bonferroni-Holm adjusted P-values calculated to determine whether time to death was significantly different between combined challenge/treatment groups. Kaplan Meier estimates were plotted for each group.
Results
PK/PD Assessment of Neupogen® (Filgrastim)
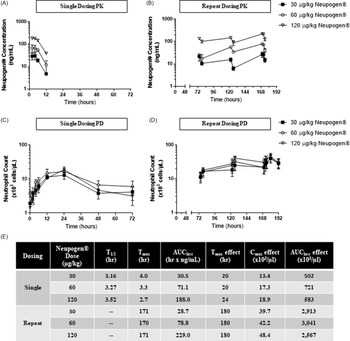
PK/PD analyses were performed in naïve rats to select the apparent human equivalent dose of Neupogen®, administered as a single dose or daily for 8 d (shown in Figure 1A and 1B). Repeat dosing had blood collections started at 72 h due to Institutional Animal Care and Use Committee (IACUC) blood collection limits, maximizing the data collected for both Neupogen® and neutrophils. The t1/2 for Neupogen® was ∼3 h, and drug concentrations did not increase with repeat dosing. PD analyses showed that 8 daily doses resulted in neutrophil counts as much as 32-fold higher than pre-dose values and 2- to 4-fold higher than a single dose (shown in Figure 1C and 1D). A summary of select PK/PD parameters for each dose group is shown in Figure 1E. Data were then compared with the serum Cmax value of 49 ng/mL in humans following a SC dose of 11.5 µg/kg Neupogen®. 21 To scale down for the recommended dosing regimen of 10 µg/kg/d following radiation injury, Reference DiCarlo, Horta and Aldrich22 the serum Cmax concentration following a 10 µg/kg injection of Neupogen® was estimated to be 42.6 ng/mL. Using the rat PK data, compartmental analysis on the group mean profiles was then performed to estimate the parameters of V_F (apparent volume of distribution), K01, and K10. With these values, simulations were then run to determine the dose in rats needed to achieve a Cmax of 42.6 ng/mL (the estimated levels in humans following a 10 µg/kg injection). This analysis estimated that an SC dose of 41.0 µg/kg of Neupogen® delivered to rats would achieve the same Cmax as humans receiving a SC dose of 10.0 µg/kg of Neupogen®. Given that daily repeat dosing did not increase drug levels, the dose level of 41 µg/kg/d of Neupogen® was used for efficacy testing, thereby lending increased human dosing relevance to any observed potential therapeutic benefits.

Figure 1. Single and repeat Neupogen® dosing in naïve Sprague-Dawley rats. Sprague-Dawley rats were administered Neupogen® subcutaneously at 30, 60, or 120 µg/kg either as a single dose or repeat daily doses for 8 d. Blood was collected and analyzed for drug levels (A and B) and neutrophil counts (C and D). Each point represents the group mean ± SD. N = 3 animals per dose level for each dosing frequency and for each timepoint. (E) A summary table of notable PK/PD parameters.
Outcomes of Neupogen® (Filgrastim) Treatment Following SM Exposure
Three testing groups were studied with 16 animals in each group: (1) vehicle challenged/vehicle treated (negative control), (2) SM challenged/vehicle treated (positive control), and (3) SM challenged/Neupogen® treated (test article). SM challenge was performed at 2.11 mg/kg which represented a revised LD50 estimated based on continued work from previous efforts. Reference Beske, Wilhelm and Harvilchuck20 Treatment was initiated at approximately 1 d after challenge. Relative to the day of challenge (Day 0), blood collections for CBCs were performed on the following days: -1, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 12, and 14.
For animals challenged with SM and treated with 5% dextrose vehicle, 3 out of 16 animals (19%) died before the study termination point. For animals challenged with SM and treated with Neupogen®, 2 of 16 (13%) animals died before the study termination point. Statistical analysis of survival outcomes and time-to-death did not find significant differences between SM-challenged animals receiving vehicle or Neupogen® treatment (shown in Figure 2A).

Figure 2. Mortality, body weight, and body temperature following intravenous SM challenge and treatment with Neupogen®. After intravenous challenge with vehicle or 2.11 mg/kg SM followed by daily subcutaneous treatment with vehicle or Neupogen® starting ∼24 h after challenge: (A) Kaplan-Meier plot of mortality; (B) body weight loss and recovery; (C) body temperature. Each point represents the group mean ± SEM with an n = 16 animals challenged per group (on Day 0). Statistical key: # indicates P < 0.05 between SM/vehicle and SM/Neupogen®; ^ indicates P < 0.05 between vehicle/vehicle and SM/Neupogen®; * indicates P < 0.05 between vehicle/vehicle and SM/vehicle.
Toxic signs observed in SM-challenged animals included diarrhea, hunched posture, roughed hair coat, lethargy, and respiratory abnormalities (labored breathing). Toxic signs observed in SM-challenged vehicle controls generally resolved by Day 12, while toxic signs for Neupogen®-treated animals resolved more rapidly by Day 9 as all animals were observed as normal.
For animals challenged with SM and treated with Neupogen®, initial body weight loss was similar to SM-challenged controls with a steady decrease in body weight through Day 5 (shown in Figure 2B). Despite similar initial weight loss, SM-challenge animals receiving Neupogen® treatment recovered body weight more rapidly and were statistically indistinguishable from vehicle-challenged controls on Days 13 and 14 while SM-challenged controls remained significantly decreased.
Body temperature fluctuations were less drastic for SM-challenged animals receiving Neupogen® treatment, with the notable absence of a sharp temperature spike on Days 6 and 7 compared with SM-challenged controls (shown in Figure 2C).
Hematologic Effects of Neupogen® (Filgrastim) Treatment Following SM Exposure
SM challenge resulted in changes to red cell and platelet parameters including red blood cell counts, reticulocyte counts, and platelet counts (shown in Figure 3A-C, respectively). Given the specific mechanism of action for Neupogen®, daily treatment did not alter these hematology parameters after SM challenge as anticipated.

Figure 3. Neupogen® does not alter red blood cell or platelet parameters following IV SM challenge. SM (2.11 mg/kg) or vehicle was administered intravenously to male Sprague-Dawley rats that received post-exposure treatment with vehicle or Neupogen®. Blood was collected at the indicated time-points for CBC analysis. Each point represents the group mean ± SEM. Blood collections at individual timepoints for each group were split among animals with an n = 16 animals challenged per group (on Day 0), ultimately ranging in a sample size of between n = 3 and n = 16 at each time-point for each group due to all animals not having blood collected at all timepoints due to IACUC sample volume limits. The following CBC parameters are presented: (A) red blood cell counts, (B) reticulocyte counts, and (C) platelet counts. Statistical key: # indicates P < 0.05 between SM/vehicle and SM/Neupogen®; ^ indicates P < 0.05 between vehicle/vehicle and SM/Neupogen®; * indicates P < 0.05 between vehicle/vehicle and SM/vehicle.
However, an effect with Neupogen® treatment was observed on white blood cell (WBC) parameters. One day after SM challenge, before receiving the first Neupogen® treatment dose, total WBCs (shown in Figure 4A) were depressed driven by a large drop in lymphocyte counts (early onset lymphopenia; shown in Figure 4B). On Day 2 after SM exposure, ∼20 h after receiving the first Neupogen® dose on Day 1, total WBC counts for Neupogen®-treated animals were statistically increased as a result of an ∼4-fold increase in neutrophil counts (shown in Figure 4C). Early neutrophilic leukocytosis has been reported in humans following dermal SM exposure Reference Sezigen, Eyison and Ortatatli5 but to date has not been observed in the rat IV SM model. As early leukocytosis was not observed in SM-challenged animals receiving vehicle treatment, the increase in neutrophil counts was considered specifically attributed to Neupogen® treatment and hypothesized to be from neutrophil de-margination into the peripheral blood and accelerated stimulation of available progenitors. Lymphocyte counts were unaffected, which caused a significant shift in neutrophil/lymphocyte ratios (shown in Figure 4D). However, the early neutrophil increase was transient as neutrophil counts (and total WBC counts) were similar to SM-controls by Day 3, followed by a similar delayed onset neutropenia at Day 4. From this nadir and in contrast to SM-controls, Neupogen®-treated animals rapidly recovered neutrophil counts by Day 6 with group mean counts statistically indistinct from vehicle-challenged controls. Neutrophil counts for Neupogen®-treated animals were statistically higher than SM-challenged controls on Days 7, 8, 9, and 10, reaching a zenith ∼10-fold higher compared with vehicle-controls on Day 10 (the day of treatment withdrawal). From the zenith, neutrophil counts decreased through Days 12 and 14 but still remained elevated compared with vehicle-challenged controls, indicating some measure of delayed reversibility after Neupogen® washout.

Figure 4. Neupogen® accelerates recovery from SM-induced neutropenia. SM (2.11 mg/kg) or vehicle was administered intravenously to male Sprague-Dawley rats that received post-exposure treatment with vehicle or Neupogen®. Blood was collected at the indicated time-points for CBC analysis. Each point represents the group mean ± SEM. Blood collections at individual timepoints for each group were split among animals with an n = 16 animals challenged per group (on Day 0), ultimately ranging in a sample size of between n = 3 and n = 16 at each time-point for each group due to all animals not having blood collected at all timepoints due to IACUC sample volume limits. The following CBC parameters are presented: (A) white blood cell counts, (B) lymphocyte counts, (C) neutrophil counts, and (D) neutrophil/lymphocyte ratio. Statistical key: # indicates P < 0.05 between SM/vehicle and SM/Neupogen®; ^ indicates P < 0.05 between vehicle/vehicle and SM/Neupogen®; * indicates P < 0.05 between vehicle/vehicle and SM/vehicle.
Discussion
The current study was undertaken to evaluate the efficacy of filgrastim, specifically Neupogen®, in a recently published rodent model of SM hematologic toxicity. Reference Beske, Wilhelm and Harvilchuck20 Although this IV model of SM intoxication is not representative of a real-world exposure scenario, the temporal progression of hematologic toxicity following SM exposure observed in IV challenged rats aligns with clinical findings, characterized by an early-onset lymphopenia followed by a delayed-onset neutropenia and thrombocytopenia. Reference Sezigen, Eyison and Ortatatli5 Consequently, monitoring potential treatment-induced changes in immune cell populations and/or resultant clinical symptoms after SM exposure in this model provides a platform to assess effectiveness of candidate MCMs.
Before evaluating the efficacy of Neupogen®, PK/PD analyses were performed in naïve Sprague-Dawley rats following either a single or daily SC dose for 8 consecutive days. Plasma drug levels were then used to estimate a human equivalent dosing regimen in rats that approximates the 10 µg/kg/d Neupogen® treatment regimen indicated for patients exposed to myelosuppressive doses of radiation. Reference DiCarlo, Horta and Aldrich22 Based on PK modeling, 41 µg/kg/d of Neupogen® in rats was calculated to replicate the human equivalent dose and was selected for the efficacy evaluation.
Although the route of SM exposure used in this study is not reflective of a realistic scenario for reasons described earlier, a real-world relevant treatment approach was used. Administration of the candidate MCM was not initiated until 1 d after intoxication when symptoms of SM poisoning typically first become apparent. More specifically, this 24-h delay in treatment coincides with evidence of early-onset lymphopenia. In addition to the severity of early symptoms of SM exposure (erythema, bilateral conjunctivitis, blisters, etc.), characteristic early changes in CBC profiles (lymphopenia, reticulocyte loss, etc.) could be used to guide treatment decisions on starting G-CSF therapy.
Daily Neupogen® treatment was determined to be capable of mitigating some critical aspects of SM-induced hematological toxicity. This conclusion is supported by the accelerated recovery of neutrophils levels in the complete blood cell count profiles obtained from SM-challenged Neupogen®-treated animals. The duration of SM-induced neutropenia was significantly reduced in animals receiving Neupogen® treatment when compared with SM controls. Consistent with the accepted mechanism of action for Neupogen®, the effect of treatment on CBC profiles was largely restricted to neutrophils, as other cell populations including red blood cells, platelets, and lymphocytes did not show a response to daily Neupogen® administration.
Of interest, an early response to Neupogen® treatment was elicited 2 d after SM challenge, a time-point which represented ∼20 h after animals received the first Neupogen® treatment. However, this early response was transient and did not have long-lasting implications as neutrophil counts for Neupogen®-treated animals were indistinguishable from SM-controls on Day 3 and went on to reach similarly decremented levels on Days 4 and 5. It is unclear if earlier post-exposure intervention with Neupogen® would provide additional benefit and/or alter neutropenic progression. A sustained response to Neupogen® treatment in SM-challenged animals was not observed until Day 6. Collectively, these data suggest that, early in the SM-induced toxicity process, there is some capacity for the bone marrow to respond to Neupogen® treatment. If the ability of Neupogen® to stimulate a bone marrow response is used as an indirect metric of injury, these data could suggest that injury to the bone marrow is an ongoing process through Days 4 and 5 before some form of regeneration/recovery occurs. Additionally, these data suggest that at 3-5 d following injury, the bone marrow may be largely refractory to any G-CSF treatments.
While daily Neupogen® treatment appeared to exert a positive effect on other endpoints of interest, including more rapidly resolving clinical signs, accelerating recovery from body weight loss, and preventing a spike in body temperature, survival rates between treatment groups were not statistically significant. However, this analysis was complicated by a lower than expected mortality rate in the SM-control groups. Despite a target challenge level of 1xLD50, only 3 of 16 animals (19%) in the SM-control group succumbed to challenge before the scheduled euthanasia date. Of note, due to surgical scheduling/animal availability, the vendor for Sprague-Dawley rats in this study was different from that used in previous model development work. Reference Beske, Wilhelm and Harvilchuck20 While analysis of baseline CBC profiles between vendor sources revealed significant differences (data not shown), most notably higher baseline WBC, lymphocyte, and neutrophil counts in rats obtained from Charles River Laboratories (CRL), it is unclear if this contributed to the lower mortality observed in this study using Sprague-Dawley rats sourced from CRL. Regardless, further study will be required to better define appropriate SM challenge levels that provide a reliable lethal background in vehicle-treated animals from which to measure countermeasure effectiveness. It is also unclear if specifically boosting a single blood cell population will provide sufficient protection from lethality if death is used as a primary read-out of efficacy. In the case of Neupogen®, given the previous model development studies where death typically occurred between 4 and 8 d postchallenge, and given that the beneficial effect of Neupogen® treatment was not apparent until at least 6 d after challenge, it may be difficult to disambiguate potential survival efficacy from early treatment-refractory death and late treatment-dependent survival.
Limitations
Potential limitations of this study include an SM exposure route (IV) that is unlikely to be representative of a human exposure, inherent limitations of using a rodent animal model for translation to humans, and the unexpected low mortality rate when challenging at a previously determined dose that resulted in 50% lethality to the animals.
Conclusions
Collectively, under the conditions of the previously published rat IV SM testing platform, daily SC post-exposure treatment with 41 µg/kg/d was effective in mitigating the duration of SM-induced neutropenia and improving other endpoints related to overall animal health. The data reported here expand upon those reported in a previous pilot study by Anderson et al. Reference Anderson, Holmes and Lee17 and further support the potential effectiveness and repurposing of Neupogen®.
Acknowledgments
The authors thank Phyllis Herr-Calomeni, Laura Hines, Jay Bruskotter, Leah Aleshire, Bradley Brown, Katie Albanese, Beth Reed, Kurt Smith, for their exceptional technical skill at Battelle; Dave West and Julianne Bartz for their thorough quality control review; Cindy Landgren for overall program management, Kemla Siddoway for management assistance, and the NIAID Radiation and Nuclear Countermeasures Program, in particular Drs. Andrea DiCarlo-Cohen (Director) and David R. Cassatt (Program Officer), for their expertise and guidance.
Author contributions
Phillip H. Beske, PhD, DABT: Study design development, study director for study, study execution, analysis of all data, drafting of manuscript. Jill A. Harvilchuck, PhD, DABT: Study design development, principal investigator for study, review of results, review/edit of manuscript. Seth T. Gibbs, PhD: Generation of PK data and analysis. Carol E. Green, PhD, DABT: PK/PD study design, execution, and analysis of PK/PD data, review/edit of manuscript. Lalitha Iyer: PK/PD study design, execution, and analysis of PK/PD data, review/edit of manuscript. Kathleen O’Loughlin: PK/PD study design, execution, and analysis of PK/PD data, review/edit of manuscript. Tom C.-C. Hu, PhD: Development of study concept, study design, review of results, review/edit of manuscript. Michael S. Nealy, PhD: Development of study concept, study design, review of results, review/edit of manuscript. Gennady E. Platoff Jr., PhD: Development of study concept, study design, review of results, review/edit of manuscript. David T. Yeung, PhD: Development of study concept, study design, review of results, review/edit of manuscript.
Funding
This work was supported by the NIH Chemical Countermeasures Research Program (CCRP) at the NIAID and the NIH Office of the Director through interagency agreements (AOD17032-001 and AOD18012-001) between the NIH and HHS/PSC and prepared under the auspice of the NIH, Contracts No. HHSP233201500095I/HHSP23337001T (PSC180304) and HHSP233201500041I/ HHSP23337003T (PSC217749). The authors declare no conflicts of interest.
Disclaimer
The views expressed are those of the authors and do not represent the official policy of Battelle, AmplifyBio, SRI International, or the US Government. No official support or endorsement of this article by Battelle, AmplifyBio, SRI International, or the U.S. Government is intended or should be inferred.