Introduction
Sleep disturbance can promote the risk of dementia and white matter lesions. For example, insomnia can increase the risk of Alzheimer’s disease (AD), and obstructive sleep apnea increases the incidence of all-cause dementia.Reference Cruz, García, Álvarez and Manzanero 1 Short-time duration of sleep is associated with late-onset dementia.Reference Sabia, Fayosse and Dumurgier 2 In addition, sleep time <7 hours or >10 hours/night, napping, and poor sleep patterns are associated with an increased risk of cardiovascular disease.Reference Wang, Yang, Li, Qi, Pan and Xu 3 Goldman et al found that glymphatic failure during sleep was associated with the risk of dementia,Reference Nedergaard and Goldman 4 and Hong et al found that hippocampal subfield atrophy in chronic insomnia was associated with impaired cognition.Reference Joo, Kim, Suh and Hong 5 Recently, a Rotterdam study found that cerebral hypoperfusion was associated with accelerated cognitive decline in the general population.Reference Wolters, Zonneveld and Hofman 6 A study using arterial spin labeling (ASL) magnetic resonance imaging (MRI) on dementia with Lewy bodies and AD found that increased perfusion was related to functional compensation and decreased perfusion was related to functional impairments.Reference Roquet, Sourty, Botzung, Armspach and Blanc 7
Currently, 54% of cerebral small vessel disease (CSVD) patients meet the standard of chronic insomnia, which is much higher than healthy elderly people.Reference Huang, Duncan, Cistulli, Nassar, Hamer and Stamatakis 8 In a previous clinical study, the researchers found that non-respiratory sleep fragmentation was associated with the cognitive dysfunction of CSVD patients, especially executive function and delayed recall ability.Reference Wang, Chen and Liao 9 However, in the CSVD population, there are still actually a few studies regarding the prevalence, characteristics, pathophysiology, effects on cognitive function, association with imaging markers, and optimal treatment strategies for insomnia.
We aim to investigate the associations between insomnia, cerebral perfusion, and cognitive impairment in patients with CSVD imaging markers and to explore the preventive strategy for dementia.
Materials and methods
Patient enrollment and study design
This is an observational cross-sectional study. Consecutive patients with symptoms and imaging characteristics of CSVD were screened and selected from the outpatient department of neurology, Huashan Hospital, Fudan University between January 2021 and March 2022. Patient enrollment was conducted according to the inclusion criteria and exclusion criteria as follows:
Inclusion criteria: Persons (Reference Cruz, García, Álvarez and Manzanero1) Aged between 50 and 85 years; (Reference Sabia, Fayosse and Dumurgier2) With one or more cerebral vascular risk factors, such as hypertension, diabetes, hyperlipidemia, and smoking; (Reference Wang, Yang, Li, Qi, Pan and Xu3) With typical characteristics of CSVDs in MRI scanning: WHMs (Fazekas 1 and above) are necessary, encephalography, subcortical infarction, perivascular space enlargement, and cerebral micro bleedings; (Reference Nedergaard and Goldman4) With symptoms involved in non-embolic lacunar stroke (unilateral motor/sensory impairment affecting at least two parts of the body (face, upper extremity, lower extremity, cognitive impairment, gait impairment, dysphagia, dysuria, and mood disorders.
Diagnostic criteria of chronic insomnia:
-
A. Dissatisfaction with sleep duration or quality.
-
B. Insomnia causes clinically obvious distress or impairment.
-
C. Sleep difficulty occurring at least 3 nights per week for at least 3 months.
-
D. Sleep difficulties occur even when there is ample opportunity for sleep.
-
E. Insomnia cannot be attributed to the physiological effects of a substance.
-
F. Co-existing mental disorders and medical conditions cannot fully explain the main complaint of insomnia.
Exclusion criteria: (Reference Cruz, García, Álvarez and Manzanero1) previous or acute large-scale cerebral infarction or watershed infarction (1.5 cm large); (Reference Sabia, Fayosse and Dumurgier2) Alzheimer’s disease, Parkinson’s disease, and other diseases that affect cognitive function; (Reference Wang, Yang, Li, Qi, Pan and Xu3) can not finish the cognitive function test, due to deafness, hemiplegia, aphasia, and visual impairment, etc.; (Reference Nedergaard and Goldman4) serious physical or neurological disorders, such as schizophrenia, bipolar disorder and serious depression. (Reference Joo, Kim, Suh and Hong5) dementia due to congenital mental disorders and other diseases.
The estimated sample size in our study was 48.Reference Wang, Chen and Liao 9
Figure 1 shows a flow diagram of patient enrollment. Written informed consents were obtained from all participants or their families. The study was approved by the Ethics Review Board of Huashan Hospital before the patient enrollment.
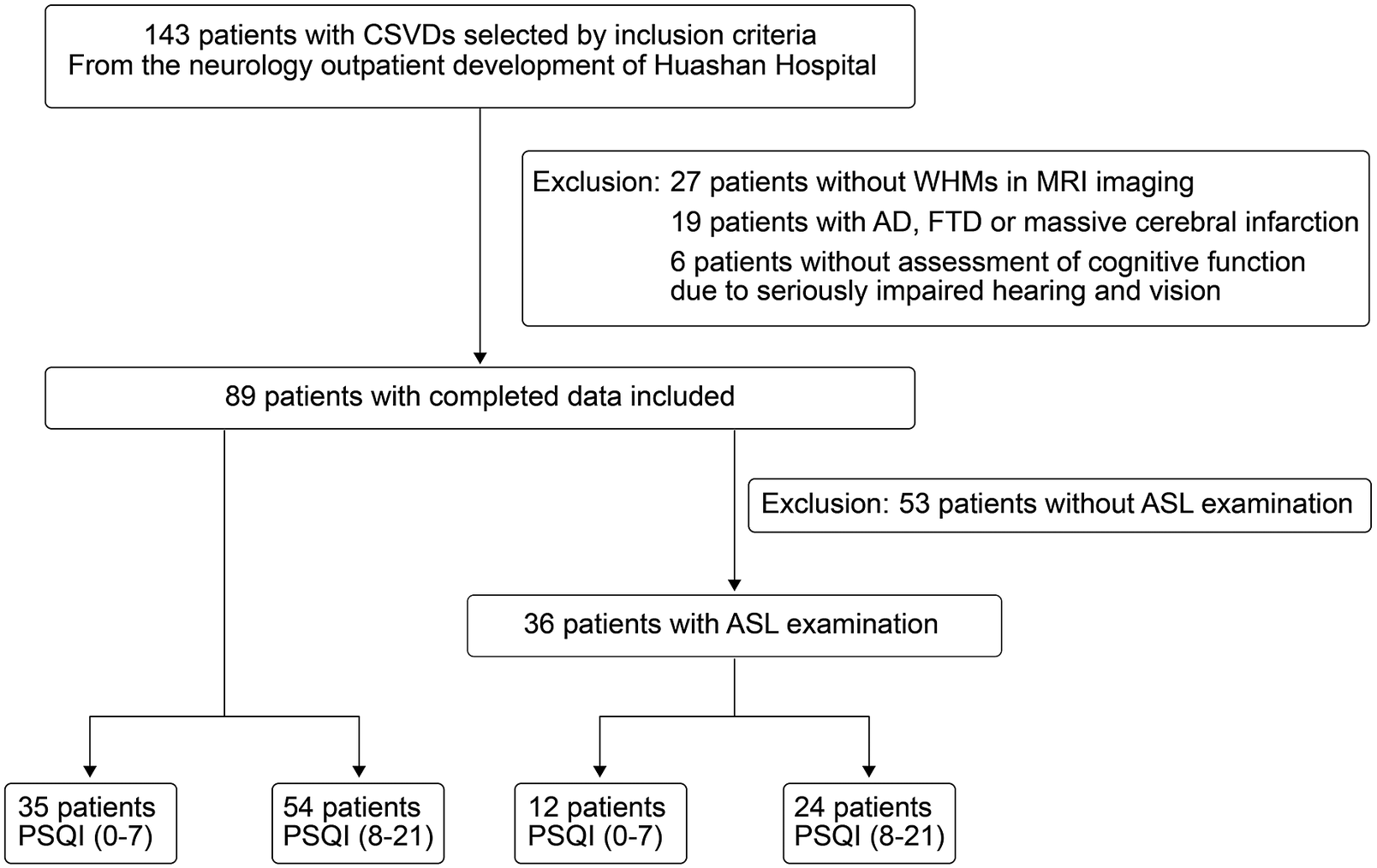
Figure 1. Flowchart of patient enrollment.
Baseline data collection
Baseline data were collected from medical records including age, sex, educational background, Hamilton Anxiety Scale (HAMA), Hamilton Depression Scale (HAMD), and history of hypertension, diabetes, coronary heart disease, atrial fibrillation, hyperlipemia, cigarette smoking, and alcohol consumption.
Clinical assessments on the sleep and cognitive function
Sleep evaluation
The Pittsburgh Sleep Quality Index (PSQI) was developed in 1993 and was suitable for domestic patients.Reference Yan, Huang, Chen, Wang, Li and Luo 10 The total score of each factor is the total score of PSQI, which ranges from 0 to 21, with a higher score indicating worse sleep quality. It is mainly used to evaluate the sleep quality of the subjects in the last month. The Insomnia Severity Index (ISI) is a supplementary scale for assessing the severity of insomnia.Reference Morin, Belleville, Bélanger and Ivers 11 The total score of ISI will be divided into 4 grades (0–7 refers to no insomnia, 8–14: mild insomnia, 15–21: moderate insomnia, 22–28: serious insomnia).
In our study, we divided patients into two groups, respectively, the normal sleep group (PSQI ≤7) and the poor sleep group (PSQI >7). Additionally, patients were also divided into 4 groups according to different insomnia symptoms in their PSQI scales, including normal sleep, difficulty in falling asleep, sleep fragmentation or early wakening, and mixed symptoms.
Cognitive function evaluation
Mini-Mental State Examination (MMSE),Reference Zhuang, Yang and Gao 12 Montreal Cognitive Assessment (Beijing) (MoCA),Reference Zhuang, Yang and Gao 12 Trail Making Test (TMT),Reference Allen, Thaler, Ringdahl, Barney and Mayfield 13 Rey-Osterrich Complex Figure Test (Rey-CFT),Reference Youn, Pyun and Ryu 14 and Chinese Auditory Verbal Learning Test (AVLT)Reference Dong, Wang, Guo, Shao, Song and Han 15 are used to evaluate patients’ cognitive performance. We defined the MoCA score 21/22 as its cut-off value in order to distinguish mild cognitive impairment from patients with CSVDs.
Imaging assessments
Fazekas grades
All the patients have completed the brain MRI scanning including T1Flair, T2WI, T2Flair (Germany, Siemens). The Fazekas scale is used to quantify the number of white matter lesions, and this classification has been proposed by Fazekas et al in 1993.Reference Fazekas, Kleinert, Offenbacher, Schmidt, Kleinert and Payer 16
ASL
Patients with CSVDs underwent a 3-dimensional ASL assessment (Germany, Siemens, Syngo MR E11). The ASL data were analyzed and processed by FSL software, and the brain tissue’s absolute perfusion value (ml/100 g/min) was calculated by calibration data. Perfusion images and structural images were combined to obtain perfusion values of different regions of the brain.
Statistical analysis
SPSS 20.0 (IBM, Armonk, NY) and Graph Prism 9.0 (GraphPad Software, San Diego, CA) were used to perform statistical analyses. Patients were dichotomized into the normal sleep group (PSQI ≤7) and poor sleep group (PSQI >7). MMSE scores, MoCA scores, and other cognitive assessment scores were tested using the Mann–Whitney U test. Cerebral blood perfusion values in the whole brain and respective regions were tested using the Students’ t-test. MoCA and MMSE scores were compared among the two groups with distinct insomnia symptoms based on PSQI, using the Kruskal-Wallis test. Binary logistic regression analysis was used to evaluate the correlation between MoCA total score and other covariables such as educational background, PSQI, ISI, hypertension, and diabetes, etc., in which MoCA scores were dichotomized with a cut-off point of 21/22 in the model. P-value was from a two-tailed test, and a P-value at .05 was considered statistically significant. Owing to one statistical test we performed, a Bonferroni correction for multiple testing was applied. The significance level P = .05 was divided by 2 (see Supplementary material Figure S2), which provides a significance level corrected for multiple testing: P = .025.
Results
Baseline characteristics and comparisons
A total of 89 patients with CSVDs were enrolled in this study and 36 patients completed the ASL. Forty-three (48%) were male; the mean age was 66.48 ± 8.12 years; and education time was 9 ± 1.5 years. Age, sex, body mass index (BMI), education level, Fazekas grade, and vascular risk factors such as hypertension, coronary heart disease/atrial fibrillation, diabetes mellitus, hyperlipidemia, smoking, and drinking did not differ significantly between the two groups. In patients with higher PSQI scores, 33 patients have hypertension, 3 have coronary heart disease/atrial fibrillation, 8 have diabetes mellitus, 23 have hyperlipidemia and 11 have smoking, 13 have alcohol-drinking, and in patients with lower PSQI scores, 24 patients have hypertension, 3 have coronary heart disease/atrial fibrillation, 2 have diabetes mellitus, 17 have hyperlipidemia and 7 have smoking, 7 have alcohol-drinking. Although the MMSE scores revealed no significant difference between the two groups (higher PSQI scores group and lower PSQI group), there was a statistically significant difference in MoCA scores. In the specific cognitive domain, there were differences in the recall (P = .0342) in MMSE, the delayed recall (P = .0289) in MoCA, recall (P = .0352) in Rey-CFT, the total score of Chinese AVLT (P = .0263) and its immediate recall score (P = .0335), short delayed score (P = .0151) and recognition discriminability (P = .0434) between the two groups (see Table 1).
Table 1. Comparisons Between the Normal Sleep Group and Poor Sleep Group in Patients With CSVDs
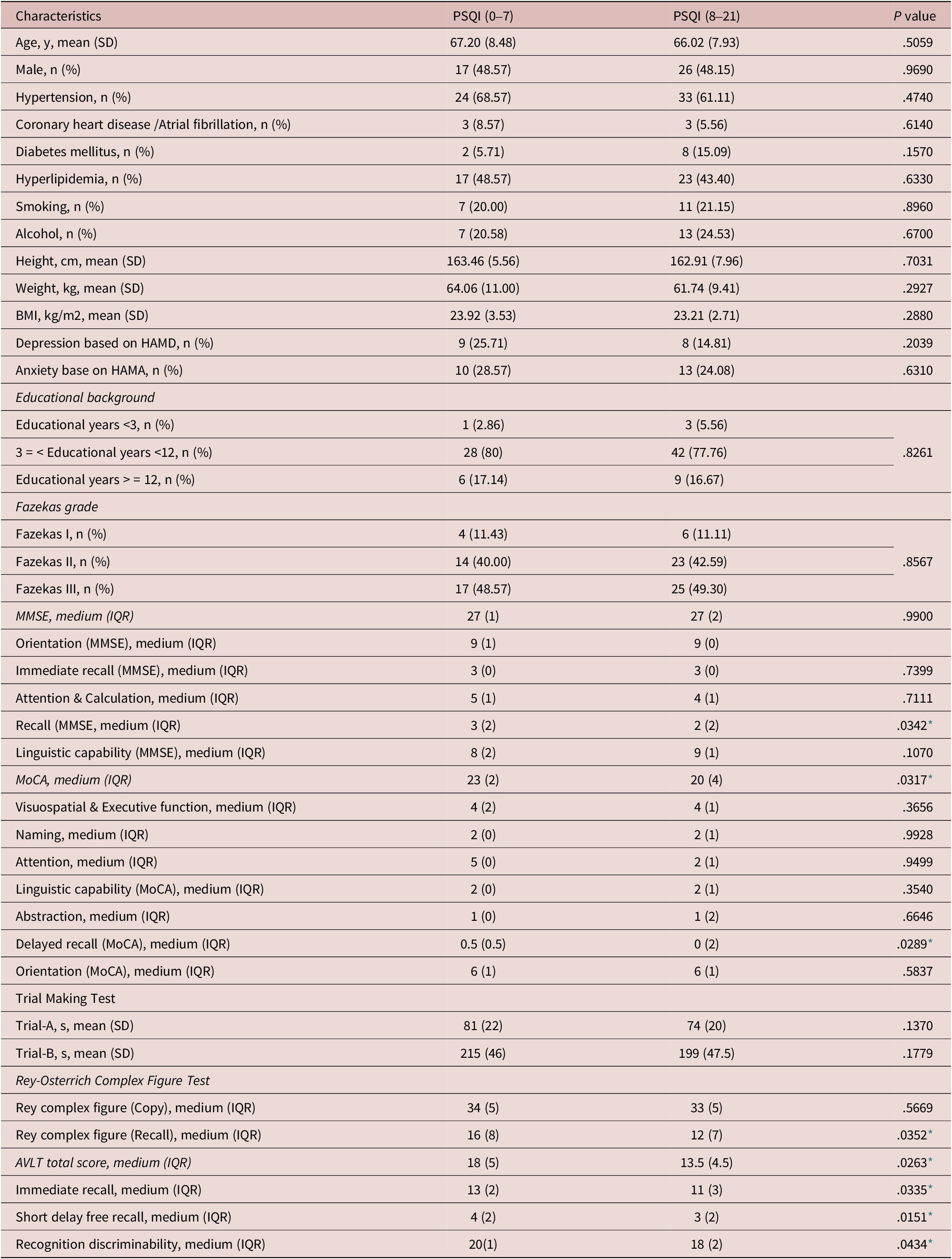
Abbreviations: AVLT, auditory-verbal learning test; CSVD, cerebral small vessel diseases; HAMA, Hamilton anxiety scale; HAMD, Hamilton depression scale; IQR, interquartile range; MMSE, mini-mental state examination, MoCA, Montreal cognitive assessment; PSQI, Pittsburgh sleep quality index; SD, standard deviation.
* statistically significant.
Associations among insomnia, cognition, and cerebral perfusion
We then divided the patients with insomnia into two subgroups according to MoCA scores, and found no significant differences in left hippocampal perfusion and also in another regional perfusion of grey matter and white matter (see Table 2).
Table 2. Comparisons Between Normal Cognition and Poor Cognition in Patients With Insomnia
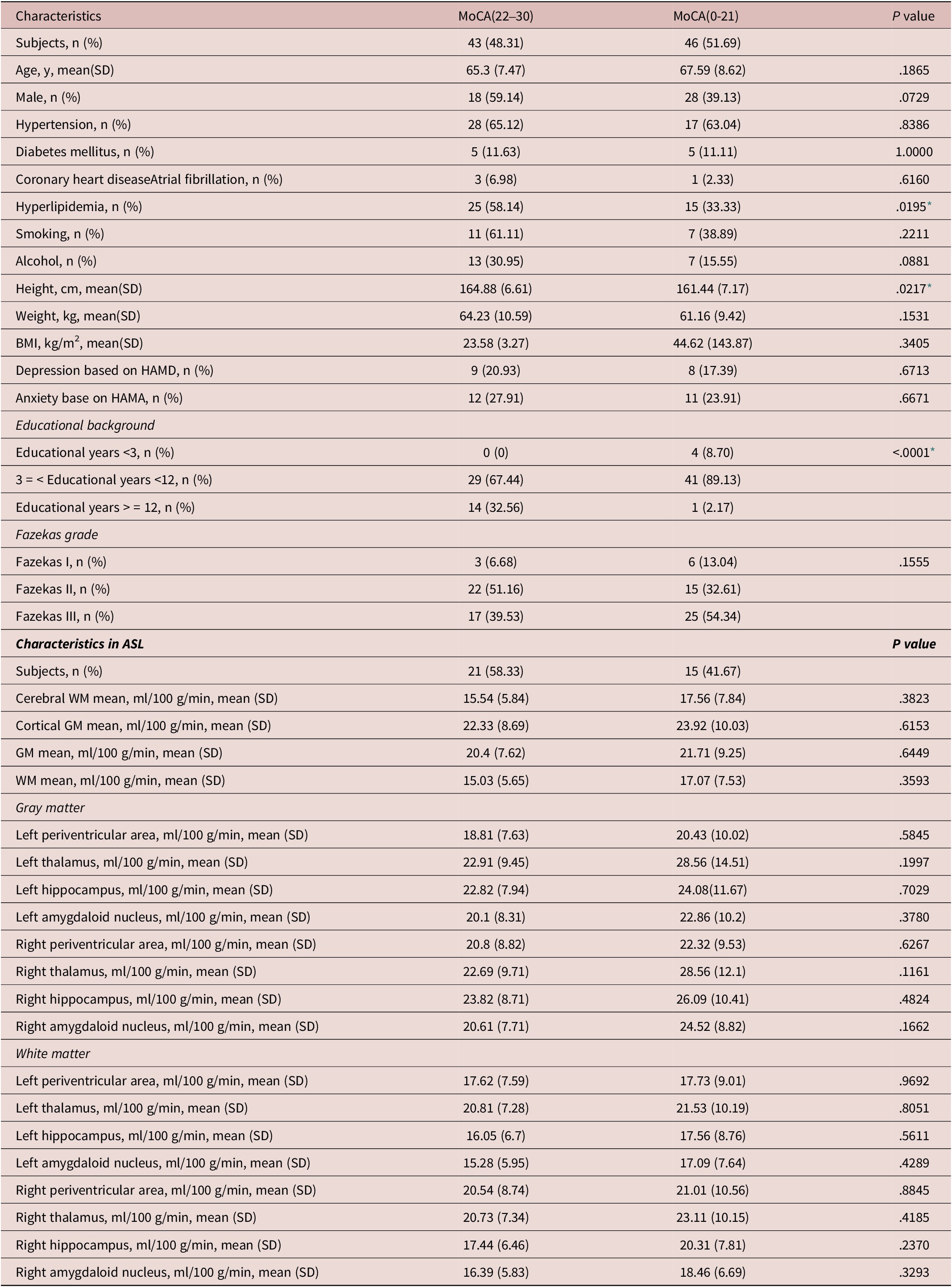
Abbreviations: ASL, arterial spin labeling; BMI, body mass index; GM, gray matter; HAMA, Hamilton anxiety scale; MoCA, Montreal cognitive assessment; SD, standard deviation; WM, white matter.
* statistically significant.
In comparisons between normal sleep and poor sleep groups, we found that left hippocampal grey matter perfusion was significantly reduced (P = .0384) in the poor sleep group and found no significant differences in other regional grey matter perfusion and white matter perfusion (see Table 3).
Table 3. Comparisons of ASL Results Between Normal Sleep Group and Poor Sleep Group in Patients With CSVDs
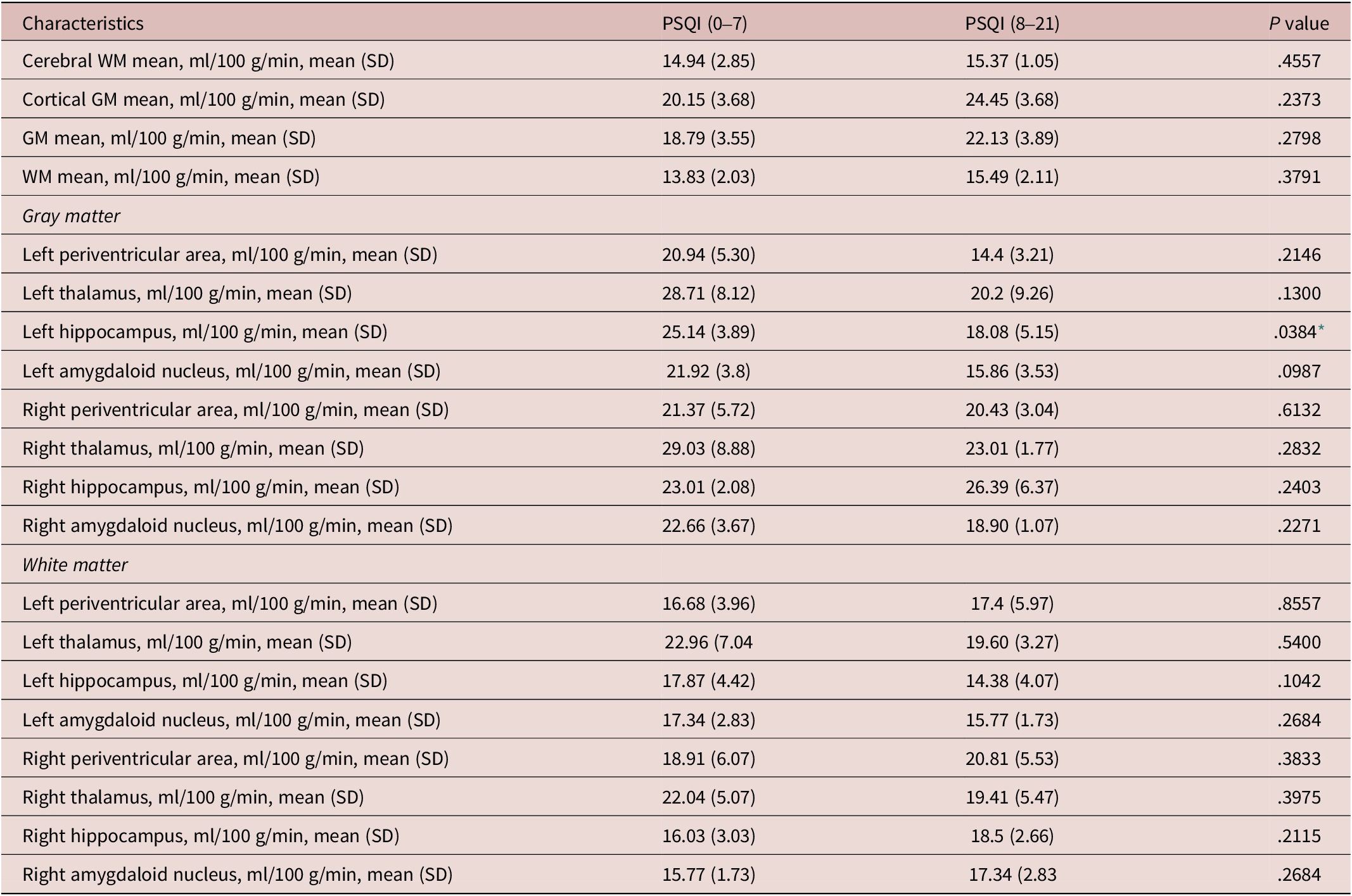
Abbreviations: ASL, arterial spin labeling; CSVD, cerebral small vessel diseases; GM, gray matter; PSQI, Pittsburgh sleep quality index; SD, standard deviation; WM, white matter.
* statistically significant.
Insomnia was independently related to cognitive decline
In the binary logistic regression analyses, considering MoCA score as the dependent variable, education background, Fazekas rating, age, and ISI score as the covariables, we found that the results showed that educational background (P < .001) and ISI score (P = .039) were independently correlated with MoCA total score (see Table 4).
Table 4. Logistic Regression Analysis

Abbreviations: ISI, insomnia severity index; MoCA, Montreal Cognitive Assessment.
* statistically significant.
Correlations between left hippocampal perfusion and insomnia
We curved the correlation between cerebral perfusion, hippocampal grey matter perfusion, and PSQI scores, and found a significant relationship between whole grey matter perfusion and the PSQI score (P = .0241, r = 0.38, 95%CI: 0.04-0.63), and significant relationship between left hippocampal perfusion and PSQI scores with P = .0065 (r = −0.045, 95%CI: [−0.68]-[−0.13]). The results showed the compensation of whole grey matter and decreased function of left hippocampal perfusion in insomniac patients (see Figure 2).
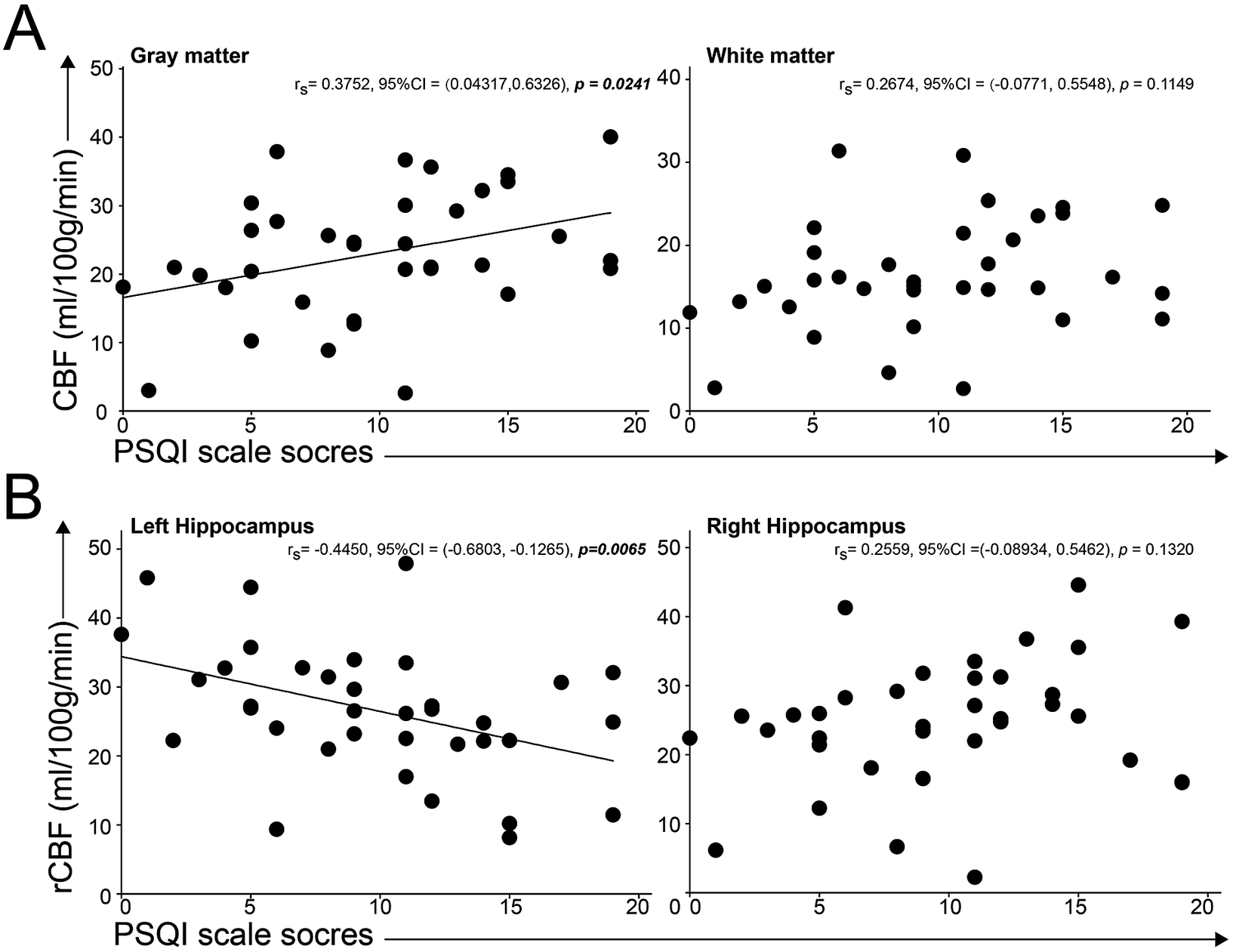
Figure 2. Correlations between cerebral perfusion and insomnia scores. A, the linear correlation between PSQI scores and CBF in grey matter was significant and the association between PSQI scores and CBF in white matter was not significant. B, we found the significant linear correlation between PSQI scores and regional CBF in left hippocampus, not in right hippocampus.
Discussion
Our study found that insomnia was associated with cognitive decline in patients with CSVD, and reduced left hippocampal perfusion was correlated with the severity of insomnia. This indicated the vital role of the left hippocampal in the pathophysiology of insomnia and cognitive impairment.
The underlying mechanism (also imaging characteristics) of insomnia was associated with cortical atrophyReference Sexton, Storsve, Walhovd, Johansen-Berg and Fjell 17 and hypertrophy. And, sleep quality and duration were associated with fractional anisotropy and mean diffusivity.Reference Sexton, Zsoldos, Filippini, Griffanti, Winkler and Mahmood 18 Alterations in grey and white matter that emerged early in development were associated with poor sleep.Reference Kocevska, Muetzel and Luik 19 In addition, the involvement of the brain circuit in sleep may support the regulation of circadian and homeostatic components.Reference Daan, Beersma and Borbély 20 However, the relationship between cerebral perfusion and insomnia was little investigated in clinical practice. Our study paid attention to the association between cerebral perfusion and insomniac disorder and found a negative correlation between insomnia and hippocampal perfusion.
The role of regional or hippocampal perfusion in sleep disorders needs a clear explanation. In a normal sleep-wake cycle, deep or slow wave sleep was characterized by a global reduction in cerebral blood flow (CBF), such as a reduction in thalamic and limbic perfusion. While rapid eye movement (REM) sleep was characterized by the activation of limbic areas including activation of hippocampal perfusion.Reference Braun, Balkin, Wesenten, Carson, Varga and Baldwin 21 In non-REM sleep, hippocampal activity did not change compared with wakefulness, which suggested the homeostatic or restorative role of neuronal glycogen in non-REM.Reference Benington and Heller 22 And, in non-REM sleep, a profound activation of the limbic core (hippocampus and amygdala) could be related to rich contents of dreams and memory formation.Reference Smith 23 , Reference Crick and Mitchison 24 Our study found that the left hippocampal perfusion was decreased in insomnia or sleep deprivation which not only led to the deactivation of the hippocampal in non-REM and REM sleep and damaged memory formation and neuronal glycogen restoration. The difference between right and left hemisphere dysfunction in dementia and motor disordersReference Güntürkün, Ströckens and Ocklenburg 25 was evaluated by clinical neurologists such as reduced left hemispheric perfusion in left-sided symptom dominance Parkinson’s patients.Reference Shang, Wu, Zhang, Chen, Cao and Chen 26 Our study found the left dominance low perfusion in the hippocampus was associated with sleep-related cognitive impairments.
The relationship between insomnia and sleepiness has been established. For example, daytime sleepiness was associated with cognitive decline in old people.Reference Jaussent, Bouyer, Ancelin, Berr, Foubert-Samier and Ritchie 27 Another study found that daytime sleepiness was related to a decline in attention and executive function.Reference Hishikawa, Fukui, Sato, Ohta, Yamashita and Abe 28 Also, executive functions and processing speed were associated with the cortical blood flow alterations in CSVDs.Reference Peres, De Guio, Chabriat and Jouvent 29 Declined CBF in the whole brain may result from white matter hyperintensity (WMH) in patients with CSVDs and there was a negative correlation between CBF and WMHs.Reference Shi, Thrippleton and Makin 30 Our study implied that insomnia symptoms might be related to decreased perfusion of gray matter in the left hippocampus in those with CSVDs and WMHs, and this perfusion change may indirectly influence cognitive function, especially in memory.
There are some limitations to the present study. First, in this cross-sectional study, we are unable to investigate the causal relationship between cognitive impairment and insomnia in patients with CSVDs and WMHs. Second, there are multi-factors affecting patients’ cognitive test performance, including the CSVD markers such as cerebral microbleeds. In addition, a larger sample may be needed for sufficient statistical power to obtain significant results. Finally, a single questionnaire is not relatively enough to assess patients’ sleep quality, and more subjective questionnaire scales and even objective monitoring like polysomnography will be needed for better assessing sleep quality.
Conclusion
In conclusion, in CSVD patients with insomnia, the more obvious insomnia symptoms are, the more severe the cognitive impairment is, especially the short-term memory impairment. However, different types of insomnia symptoms based on PSQI are not related to cognitive impairment. There may be a correlation among sleep, memory, and local cerebral perfusion alteration, for example, left hippocampal gray matter, in patients with CSVDs and WMHs. And we suspected that this perfusion change might indirectly influence cognitive function, especially in memory, through poor sleep performance.
Abbreviations
- AVLT
-
auditory-verbal learning test
- CSVD
-
cerebral small vessel diseases
- HAMA
-
Hamilton anxiety scale
- HAMD
-
Hamilton depression scale
- ISI
-
insomnia severity index
- MMSE
-
mini-mental state examination
- MoCA
-
Montreal cognitive assessment
- PSQI
-
Pittsburgh sleep quality index
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S1092852923002250.
Acknowledgments
We thank the patients and their families for their support and contributions. We thank Dr. Yuanyuan Wang for her advice on Tables 1–4.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Author contribution
Substantial contributions to the conception or design of the work: Y.T.; the acquisition, analysis, or interpretation of data for the work: W.Y., D.H., Z.L., W.T.; drafting the work or revising it critically for important intellectual content: D.H., Z.L.; final approval of the version to be published: X.H., Y.T.; agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: X.H., Y.T.
Financial support
The present study was supported by grants from the National Natural Science Foundation of China (Grant No. 82171460) and Huashan Hospital, Fudan University (Grant No. 220457).
Disclosure
The authors declare none.










