Take-Home Points
1. Preoccupation with naming the condition of pathological laughing and crying can obscure the underlying pathophysiology and mechanism of effective psychopharmacological treatment of the syndrome many now call pseudobulbar affect (PBA).
2. No matter what it is called, the syndrome of PBA with pathological laughing and crying can result when any number of neurological disorders structurally disrupts cortical and/or cerebellar inputs to nerve centers that control emotional expression in the brainstem.
3. Hypothetically, functional disruption of these same brain cortical/cerebellar brain circuits may underlie not only pathological laughing and crying but also several other labile, unstable, and dysfunctional symptoms of emotional dysregulation in psychiatric disorders, such as behavioral symptoms of dementia, traumatic brain injury, post-traumatic stress disorder, borderline personality disorder, treatment refractory mood disorders, multiple impulsive-compulsive disorders, and beyond.
4. The observation that dextromethorphan-quinidine reduces the involuntary symptoms of PBA has stimulated the hypothesis that sigma-glutamate-serotonin modulation of brain circuits that regulate emotional expression could provide a novel therapeutic approach to improving symptoms in wide-ranging psychiatric disorders and their symptoms of emotional expression, just as they do in PBA.
Introduction
A rose by any other name would smell as sweet. Unfortunately, that lesson from Shakespeare may have been lost when it comes to pseudobulbar affect (PBA), since the past century has seen more debate over what to call this condition than on what brain circuits are malfunctioning or how to treat it.Reference Darwin 1 , Reference Wilson 2 Table 1 lists just some of the names over the past 100 years given to a condition now called PBA and now defined rather narrowly as a neurological—not psychiatric—disorder of emotional expression characterized clinically only by frequent, involuntary, and uncontrollable outbursts of laughing and/or crying that are incongruous with or disproportionate to the patient’s emotional state and not by any other disordered symptoms of emotional expression.Reference Cummings, Gilbart and Andersen 3 – Reference Cummings 9 The nosological debate portrayed in Table 1 may be an interesting “semantic minefield,” but it also may distract attention from using the lessons of PBA to define a broader set of related conditions hypothetically linked to the same disordered brain circuits as PBA, and thus hypothetically treatable by the same psychopharmacologic approach that is effective in PBA.
Table 1 The semantic minefield of pseudobulbar affect

Where Is the Lesion in PBA?
Using the current terminology and definition of PBA, pathological laughing and crying appear to be determined by anatomic location of brain lesions independent of the underlying cause, which can be quite diverse (Table 2).Reference Cummings, Gilbart and Andersen 3 – Reference Cummings 9 This leads to 2 questions:
1. Do these lesions cause more symptoms of disordered emotional expression in PBA patients than just pathological laughing and crying (Table 3)?
2. Are psychiatric conditions (Table 4) with symptoms of disordered emotional expression (Table 3) similarly linked to the same brain circuits involved in PBA and thus potentially treatable by the same psychopharmacologic approach proven effective for PBA?
Table 2 6 classical neurological conditions with disordered emotional expression manifesting as pseudobulbar affect

Table 3 Beyond pseudobulbar affect: symptoms of disordered emotional expression

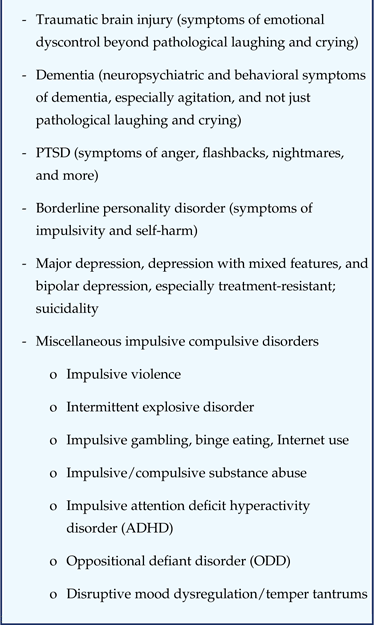
Table 4 Potential psychiatric conditions with disordered emotional expression due to dysfunction of the same brain circuits underlying pseudobulbar affect: loss of top-down control of bottom-up emotional drives
Specifically, the pathophysiology of PBA is thought to involve injury to the brain circuits that regulate the expression of affect.Reference Cummings, Gilbart and Andersen 3 – Reference Parvizi, Anderson, Martin, Damasio and Damasio 11 That is, PBA is theoretically the result of brain lesions caused principally by 6 neurological disorders (Table 2) known to damage pathways in the frontal lobes and descending (“top-down”) to the brain stem, basis pontis, and cerebellum, together comprising systems thought to be involved in motor control of emotional expression.Reference Cummings, Gilbart and Andersen 3 – Reference Parvizi, Anderson, Martin, Damasio and Damasio 11 Identifying the “lesion” and its neuroanatomical site is a rather old-fashioned way of defining a neurological disorder. A more modern viewpoint is the dimensional perspective that links individual symptoms to inefficient information processing in discrete brain circuits while determining the neurotransmitters, genomics, and drugs that can impact these circuits and improve functioning.Reference Insel, Cuthbert and Garvey 12 – Reference Stahl 14 For PBA, the dimensional approach identifies as the critical brain circuits the top-down pathways that control a whole range of emotions and their expressions beyond pathological laughing and crying and in numerous disorders beyond the classical 6 disorders known to cause PBA (Table 2).Reference Stahl 14 – Reference Everitt and Robbins 22 Numerous neurotransmitters and genes regulate these top-down pathways, including glutamate, sigma, and serotonin inputs among others, although the exact role of neurotransmitters, genomics, and drugs in the top-down pathways is just beginning to be clarified.Reference Stahl 14 – Reference Everitt and Robbins 22
The problem with paying attention exclusively to PBA as currently defined is that this may provide a barrier to its identification and treatment by mental health and other non-neurological professionals and to extending the lessons from PBA to potentially related conditions and symptoms. That is, if PBA is a “neurological” condition (Table 2) more akin to an abnormal motor reflex of facial expression than to an emotional disorder, it may lead some non-neurological professionals to be hesitant to diagnose and treat PBA; they may conclude that PBA is not in their specialty’s “wheelhouse” and think erroneously that it is not even listed in the Diagnostic and Statistical Manual of Mental Disorders of the American Psychiatric Association (23) or in the International Classification of Diseases (see Table 1 for those entries).
However, the same disorders that cause PBA symptoms of pathological laughing and crying (Table 2) may also cause numerous other symptoms of emotional expression (Table 3)Reference Darwin 1 – Reference Cummings 9 ; these additional symptoms of emotional expression (Table 3) are also seen in numerous other disorders (Table 4).Reference Stahl 14 – 23 The dimensional approach to symptomsReference Insel, Cuthbert and Garvey 12 – Reference Stahl 14 thus generates the hypothesis that the additional symptoms of emotional dysregulation beyond pathological laughing and crying (Table 3) seen in the disorders known to cause PBA also may be linked to the same top-down brain circuits thought to be compromised in PBA and therefore also potentially treatable by the same psychopharmacological approach proven effective for the narrowly defined classic neurologic symptoms of PBA (Table 3). Furthermore, the dimensional approach also generates the hypothesis that these same additional symptoms of emotional dysregulation (Table 3) seen in numerous other psychiatric disorders (Table 4) may be linked to those disordered top-down PBA circuits and therefore also treatable by PBA psychopharmacology, such as dextromethorphan-quinidine.Reference Stahl 14 – Reference Everitt and Robbins 22
Is Dextromethorphan-Quinidine–Responsive PBA a Psychopharmacological Model for Wide-Ranging Disorders of Emotional Expression?
It is plausible, but yet unproven, that PBA is a disorder of top-down brain circuits, and that symptoms of abnormal emotional expression (Table 3) in disorders of emotional dyscontrol (Table 4) result from inefficient information processing in overlapping brain circuits, the same as those hypothesized to be damaged in PBA.Reference Parvizi, Coburn, Shillcutt, Coffey, Lauterbach and Mendez 10 , Reference Parvizi, Anderson, Martin, Damasio and Damasio 11 , Reference Stahl 14 – Reference Everitt and Robbins 22 Much further work must be done in order to demonstrate that the areas of overt neurological damage or injury in PBA are in the same sites as “functional” disarray of circuits hypothesized to cause inefficient information processing, resulting in emotional dyscontrol in numerous psychiatric disorders.Reference Stahl 14 – Reference Everitt and Robbins 22 Furthermore, much further work must be done to study the symptoms beyond pathological laughing and crying (Table 3) in the neurological conditions known to cause PBA (Table 2), and also in the psychiatric disorders associated with symptoms of emotional dyscontrol (Table 4).Reference Stahl 14 – Reference Everitt and Robbins 22
Dextromethorphan’s mechanism of therapeutic action theoretically combines activity at sigma, glutamate, and serotonin sites.Reference Stahl 24 It is also plausible that dextromethorphan-quinidine–responsive PBA may serve as a psychopharmacological model for related symptoms of emotional control in a wide range of neurological and psychiatric disorders. For example, some evidence already exists that dextromethorphan-quinidine may be effective in reducing the irritability associated with Alzheimer dementia.Reference Cummings, Lyketsos and Tariot 25 Anecdotal evidence exists for effectiveness of dextromethorphan-quinidine in other non-pathological laughing and crying symptoms of traumatic brain injury,Reference Garcia-Baran, Johnson, Wagner, Shen and Geers 26 and in emotional lability in mood disordersReference Messias and Everett 27 , Reference Kelly and Lieberman 28 and post-traumatic stress disorder (PTSD). However, far more studies including randomized, controlled trials are necessary to exploit the promise of the model of dextromethorphan-quinidine–responsive PBA and thus determine whether this psychopharmacologic approach can be extended to symptoms beyond pathological laughing and crying and to numerous additional psychiatric disorders characterized by symptoms of emotional dyscontrol.
Summary
PBA symptoms of pathological laughing and crying in various neurological conditions as well as symptoms of disordered emotional expression in numerous psychiatric illnesses may all result from compromise to a network of “top-down” pathways that process sensory input, emotional output, and the intensity of emotional response. Pathological laughing and crying in neurological conditions that cause PBA are proven to be effectively treated by a sigma-glutamate-serotonin psychopharmacologic approach exemplified by dextromethorphan-quinidine. Dextromethorphan-quinidine–responsive PBA theoretically can serve as both a pathophysiologic and a treatment model for diverse conditions and symptoms of disordered emotional expression.








