No CrossRef data available.
Article contents
Extremely Al-Depleted Chlorites From Dolomite Carbonatites of the Kovdor Ultramafic-Alkaline Complex, Kola Peninsula, Russia
Published online by Cambridge University Press: 01 January 2024
Abstract
The problem to be solved is whether Al is a necessary component of Fe-Mg chlorites. Very unusual Al-depleted and Fe-enriched trioctahedral chlorites with the empirical formulae Na0.05Ca0.05(Fe2+3.01Mg2.01Ti0.14Fe3+0.04)Σ6.00[(Si3.53Fe3+0.41Al0.06)Σ4.00O10](OH)8·nH2O (Sample 1) and Na0.05Ca0.01(Fe2+3.26Mg1.97Fe3+0.75Mn0.01Ti0.01)Σ6.00[(Si3.16Fe3+0.75Al0.09)Σ4.00O10](OH)8 (Sample 2) have been discovered in Al-depleted dolomite carbonatites of the Kovdor complex of ultramafic, alkaline rocks and carbonatites, Kola Peninsula, Russia. The presence of substantial amounts of Ti in Sample 1 is another unusual feature of this mineral. In both samples, chlorites are intimately intergrown with cronstedtite-1T which is an indication of a low stability of chlorite structure in the absence of aluminum in the tetrahedral sheet. The crystal structure of chlorite in Sample 1 was solved by the Rietveld method. The mineral is triclinic (IIb-4-module), space group C-1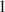 , a = 5.4153(4), b = 9.3805(7), c = 14.5743(12) Å, α = 90.137(5)°, β = 96.928(5)°, γ = 90.043(6)°, V = 734.95(10) Å3, and Z = 2. A problem to be solved is how stable are Al-free chlorites belonging to the clinochlore–chamosite solid-solution series and whether their existence in natural mineral assemblages is possible. The results obtained indicate that even though Al-depleted chlorites belonging to the clinochlore–chamosite solid-solution series exist in Nature as metastable phases, these minerals are extremely rare and much less stable than Al-poor serpentines.
, a = 5.4153(4), b = 9.3805(7), c = 14.5743(12) Å, α = 90.137(5)°, β = 96.928(5)°, γ = 90.043(6)°, V = 734.95(10) Å3, and Z = 2. A problem to be solved is how stable are Al-free chlorites belonging to the clinochlore–chamosite solid-solution series and whether their existence in natural mineral assemblages is possible. The results obtained indicate that even though Al-depleted chlorites belonging to the clinochlore–chamosite solid-solution series exist in Nature as metastable phases, these minerals are extremely rare and much less stable than Al-poor serpentines.
Keywords
- Type
- Article
- Information
- Copyright
- Copyright © Clay Minerals Society 2020




