Article contents
Adsorption of AUCL4- by Kaolinites: Effect of pH, Temperature and Kaolinite Crystallinity
Published online by Cambridge University Press: 01 January 2024
Abstract
Adsorption of the AuCl4−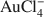 complex by kaolinites of different crystallinities (Kao-1 and Kao-2) from 100 mL of AuCl3·HCl·4H2O solutions containing 10,000, 500 and 50 µg/L Au and 1 g of kaolinite was measured at pH 3 to 9 at ambient temperature and 120°C. Adsorption from the 50, 500 and 10,000 µg Au/L solutions ranged from 64 to 100% at ambient temperature and from 68 to 100% at 120°C for both kaolinites. Adsorption was pH dependent with a maximum at pH <5 and a minimum at neutral and alkaline pHs. Up to 1 mg Au/g kaolinite was adsorbed by the kaolinites at both ambient temperature and 120°C. In a separate Au adsorption experiment using 100 mL of 4000 µg Au/L solutions and 0.02 to 1.0 g of Kao-1, up to 8.55 mg Au/ g of kaolinite was adsorbed. The pH dependence of Au adsorption suggests that surface complexation of Au to alumina sites at the edges of kaolinite particles might be involved. Protonation of kaolinite surface sites might facilitate adsorption of the anionic Au complex. Both kaolinites adsorbed ~100% of added Au at low pH values, but the less crystalline kaolinite (Kao-2) adsorbed more Au at high pH. Greater Au adsorption would be expected for the less crystalline Kao-2 sample if adsorption occurred at the edges of kaolinite particles.
complex by kaolinites of different crystallinities (Kao-1 and Kao-2) from 100 mL of AuCl3·HCl·4H2O solutions containing 10,000, 500 and 50 µg/L Au and 1 g of kaolinite was measured at pH 3 to 9 at ambient temperature and 120°C. Adsorption from the 50, 500 and 10,000 µg Au/L solutions ranged from 64 to 100% at ambient temperature and from 68 to 100% at 120°C for both kaolinites. Adsorption was pH dependent with a maximum at pH <5 and a minimum at neutral and alkaline pHs. Up to 1 mg Au/g kaolinite was adsorbed by the kaolinites at both ambient temperature and 120°C. In a separate Au adsorption experiment using 100 mL of 4000 µg Au/L solutions and 0.02 to 1.0 g of Kao-1, up to 8.55 mg Au/ g of kaolinite was adsorbed. The pH dependence of Au adsorption suggests that surface complexation of Au to alumina sites at the edges of kaolinite particles might be involved. Protonation of kaolinite surface sites might facilitate adsorption of the anionic Au complex. Both kaolinites adsorbed ~100% of added Au at low pH values, but the less crystalline kaolinite (Kao-2) adsorbed more Au at high pH. Greater Au adsorption would be expected for the less crystalline Kao-2 sample if adsorption occurred at the edges of kaolinite particles.
- Type
- Research Article
- Information
- Copyright
- Copyright © 2003, The Clay Minerals Society
References
- 6
- Cited by




