Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Meunier, A.
Sardini, P.
Robinet, J. C.
and
Prêt, D.
2007.
The petrography of weathering processes: facts and outlooks.
Clay Minerals,
Vol. 42,
Issue. 4,
p.
415.
Ortega-Castro, J.
Hernández-Haro, N.
Hernández-Laguna, A.
and
Sainz-Díaz, C. I.
2008.
DFT calculation of crystallographic properties of dioctahedral 2:1 phyllosilicates.
Clay Minerals,
Vol. 43,
Issue. 3,
p.
351.
Meleshyn, Artur
2008.
Two-Dimensional Ordering of Water Adsorbed on a Mica Surface at Room Temperature.
The Journal of Physical Chemistry C,
Vol. 112,
Issue. 37,
p.
14495.
Ortega-Castro, Joaquín
Hernández-Haro, Noemí
Muñoz-Santiburcio, Daniel
Hernández-Laguna, Alfonso
and
Sainz-Díaz, C. Ignacio
2009.
Crystal structure and hydroxyl group vibrational frequencies of phyllosilicates by DFT methods.
Journal of Molecular Structure: THEOCHEM,
Vol. 912,
Issue. 1-3,
p.
82.
Fritz, B.
Clément, A.
Amal, Y.
and
Noguera, C.
2009.
Simulation of the nucleation and growth of simple clay minerals in weathering processes: The NANOKIN code.
Geochimica et Cosmochimica Acta,
Vol. 73,
Issue. 5,
p.
1340.
Birgersson, Martin
and
Karnland, Ola
2009.
Ion equilibrium between montmorillonite interlayer space and an external solution—Consequences for diffusional transport.
Geochimica et Cosmochimica Acta,
Vol. 73,
Issue. 7,
p.
1908.
Meunier, Alain
Petit, Sabine
Cockell, Charles S.
El Albani, Abderrazzak
and
Beaufort, Daniel
2010.
The Fe-Rich Clay Microsystems in Basalt-Komatiite Lavas: Importance of Fe-Smectites for Pre-Biotic Molecule Catalysis During the Hadean Eon.
Origins of Life and Evolution of Biospheres,
Vol. 40,
Issue. 3,
p.
253.
Kalo, Hussein
Möller, Michael W.
Ziadeh, Mazen
Dolejš, David
and
Breu, Josef
2010.
Large scale melt synthesis in an open crucible of Na-fluorohectorite with superb charge homogeneity and particle size.
Applied Clay Science,
Vol. 48,
Issue. 1-2,
p.
39.
Lanson, Bruno
2011.
Layered Mineral Structures and their Application in Advanced Technologies.
p.
151.
Noguera, C.
Fritz, B.
and
Clément, A.
2011.
Simulation of the nucleation and growth of clay minerals coupled with cation exchange.
Geochimica et Cosmochimica Acta,
Vol. 75,
Issue. 12,
p.
3402.
Pike, W. T.
Staufer, U.
Hecht, M. H.
Goetz, W.
Parrat, D.
Sykulska-Lawrence, H.
Vijendran, S.
and
Madsen, M. B.
2011.
Quantification of the dry history of the Martian soil inferred from in situ microscopy.
Geophysical Research Letters,
Vol. 38,
Issue. 24,
p.
n/a.
Bouchez, Julien
Gaillardet, Jérôme
France‐Lanord, Christian
Maurice, Laurence
and
Dutra‐Maia, Poliana
2011.
Grain size control of river suspended sediment geochemistry: Clues from Amazon River depth profiles.
Geochemistry, Geophysics, Geosystems,
Vol. 12,
Issue. 3,
Matusik, Jakub
Wisła-Walsh, Ewa
Gaweł, Adam
Bielańska, Elżbieta
and
Bahranowski, Krzysztof
2011.
Surface Area and Porosity of Nanotubes Obtained from Kaolin Minerals of Different Structural Order.
Clays and Clay Minerals,
Vol. 59,
Issue. 2,
p.
116.
Klebow, Birthe
and
Meleshyn, Artur
2012.
Monte Carlo Study of the Adsorption and Aggregation of Alkyltrimethylammonium Chloride on the Montmorillonite–Water Interface.
Langmuir,
Vol. 28,
Issue. 37,
p.
13274.
Kalo, Hussein
Möller, Michael W.
Kunz, Daniel A.
and
Breu, Josef
2012.
How to maximize the aspect ratio of clay nanoplatelets.
Nanoscale,
Vol. 4,
Issue. 18,
p.
5633.
Carlsson, Torbjörn
Muurinen, Arto
and
Root, Andrew
2013.
NMR Study of Interlayer and Non-interlayer Porewater in Water-saturated Bentonite and Montmorillonite.
MRS Proceedings,
Vol. 1518,
Issue. ,
p.
167.
Decarreau, Alain
Petit, Sabine
Andrieux, Pauline
Villieras, Frederic
Pelletier, Manuel
and
Razafitianamaharavo, Angelina
2014.
Study of Low-Pressure Argon Adsorption on Synthetic Nontronite: Implications for Smectite Crystal Growth.
Clays and Clay Minerals,
Vol. 62,
Issue. 2,
p.
102.
Ngo, Viet V.
Delalande, Manuella
Clément, Alain
Michau, Nicolas
and
Fritz, Bertrand
2014.
Coupled transport-reaction modeling of the long-term interaction between iron, bentonite and Callovo-Oxfordian claystone in radioactive waste confinement systems.
Applied Clay Science,
Vol. 101,
Issue. ,
p.
430.
Lerouge, Catherine
Grangeon, Sylvain
Claret, Francis
Gaucher, Eric
Blanc, Philippe
Guerrot, Catherine
Flehoc, Christine
Wille, Guillaume
and
Mazurek, Martin
2014.
Mineralogical and Isotopic Record of Diagenesis from the Opalinus Clay Formation at Benken, Switzerland: Implications for the Modeling of Pore-Water Chemistry in a Clay Formation.
Clays and Clay Minerals,
Vol. 62,
Issue. 4,
p.
286.
Ebrahimi, Davoud
Whittle, Andrew J.
and
Pellenq, Roland J.-M.
2014.
Mesoscale properties of clay aggregates from potential of mean force representation of interactions between nanoplatelets.
The Journal of Chemical Physics,
Vol. 140,
Issue. 15,
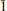 10] and [
10] and [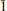
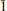 0]. The accumulation of crystal defects poisons the crystal growth along one, two or three PBCs. Then, nucleation becomes less energy-consuming than crystal growth and favours the formation of numerous smaller crystals rather than fewer bigger ones.
0]. The accumulation of crystal defects poisons the crystal growth along one, two or three PBCs. Then, nucleation becomes less energy-consuming than crystal growth and favours the formation of numerous smaller crystals rather than fewer bigger ones.



