Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Bender Koch, C.
and
Mørup, S.
1991.
Identification of green rust in an ochre sludge.
Clay Minerals,
Vol. 26,
Issue. 4,
p.
577.
Lindgreen, H.
Garnaes, J.
Besenbacher, F.
Laegsgaard, E.
and
Stensgaard, I.
1992.
Illite-smectite from the North Sea investigated by scanning tunnelling microscopy.
Clay Minerals,
Vol. 27,
Issue. 3,
p.
331.
Banin, A.
Ben‐Shlomo, T.
Margulies, L.
Blake, D. F.
Mancinelli, R. L.
and
Gehring, A. U.
1993.
The nanophase iron mineral(s) in Mars soil.
Journal of Geophysical Research: Planets,
Vol. 98,
Issue. E11,
p.
20831.
Sagoe-Crentsil, K.K.
and
Glasser, F.P.
1993.
“Green rust”, iron solubility and the role of chloride in the corrosion of steel at high pH.
Cement and Concrete Research,
Vol. 23,
Issue. 4,
p.
785.
Schwertmann, U.
and
Fechter, H.
1994.
The Formation of Green Rust and Its Transformation to Lepidocrocite.
Clay Minerals,
Vol. 29,
Issue. 1,
p.
87.
Hansen, Hans Christian Bruun
Borggaard, Ole Kragholm
and
Sørensen, Jan
1994.
Evaluation of the free energy of formation of Fe(II)-Fe(III) hydroxide-sulphate (green rust) and its reduction of nitrite.
Geochimica et Cosmochimica Acta,
Vol. 58,
Issue. 12,
p.
2599.
Blengino, J.M.
Keddam, M.
Labbe, J.P.
and
Robbiola, L.
1995.
Physico-chemical characterization of corrosion layers formed on iron in a sodium carbonate-bicarbonate containing environment.
Corrosion Science,
Vol. 37,
Issue. 4,
p.
621.
Refait, Ph.
and
Genin, J.-M. R.
1997.
Mechanisms of oxidation of Ni(II)-Fe(II) hydroxides in chloride-containing aqueous media: role of the pyroaurite-type Ni-Fe hydroxychlorides.
Clay Minerals,
Vol. 32,
Issue. 4,
p.
597.
Refait, Ph.
Drissi, S.H.
Pytkiewicz, J.
and
Génin, J.-M.R.
1997.
The anionic species competition in iron aqueous corrosion: Role of various green rust compounds.
Corrosion Science,
Vol. 39,
Issue. 9,
p.
1699.
Simon, L.
Génin, J.-M.R.
and
Refait, Ph.
1997.
Standard free enthalpy of formation of Fe(II)Fe(III) hydroxysulphite green rust one and its oxidation into hydroxysulphate green rust two.
Corrosion Science,
Vol. 39,
Issue. 9,
p.
1673.
Fredrickson, James K.
Zachara, John M.
Kennedy, David W.
Dong, Hailang
Onstott, Tullis C.
Hinman, Nancy W.
and
Li, Shu-mei
1998.
Biogenic iron mineralization accompanying the dissimilatory reduction of hydrous ferric oxide by a groundwater bacterium.
Geochimica et Cosmochimica Acta,
Vol. 62,
Issue. 19-20,
p.
3239.
Hansen, H. C. B.
and
Bender Koch, C.
1998.
Reduction of nitrate to ammonium by sulphate green rust: activation energy and reaction mechanism.
Clay Minerals,
Vol. 33,
Issue. 1,
p.
87.
Génin, Jean-Marie R.
Bourrié, Guilhem
Trolard, Fabienne
Abdelmoula, Mustapha
Jaffrezic, Anne
Refait, Philippe
Maitre, Véronique
Humbert, Bernard
and
Herbillon, Adrien
1998.
Thermodynamic Equilibria in Aqueous Suspensions of Synthetic and Natural Fe(II)−Fe(III) Green Rusts: Occurrences of the Mineral in Hydromorphic Soils.
Environmental Science & Technology,
Vol. 32,
Issue. 8,
p.
1058.
Refait, P.
Charton, A.
and
Génin, J.-M.R.
1998.
Identification, composition, thermodynamic and structural properties of a pyroaurite-like iron(II)-iron (III) hydroxy-oxalate green rust.
European Journal of Solid State and Inorganic Chemistry,
Vol. 35,
Issue. 10-11,
p.
655.
Bessière, Jacques
Perdicakis, Michel
and
Humbert, Bernard
1999.
Formation de la rouille verte II sulfatée Fe2(OH)12SO4 par oxydation du sulfate ferreux par l'eau.
Comptes Rendus de l'Académie des Sciences - Series IIC - Chemistry,
Vol. 2,
Issue. 2,
p.
101.
Hansen, Hans Christian Bruun
and
Poulsen, Inge Fiil
1999.
Interaction of Synthetic Sulphate “Green Rust” with Phosphate and the Crystallization of Vivianite.
Clays and Clay Minerals,
Vol. 47,
Issue. 3,
p.
312.
Erbs, Marianne
Bruun Hansen, Hans Christian
and
Olsen, Carl Erik
1999.
Reductive Dechlorination of Carbon Tetrachloride Using Iron(II) Iron(III) Hydroxide Sulfate (Green Rust).
Environmental Science & Technology,
Vol. 33,
Issue. 2,
p.
307.
Roh, Y.
Lee, S. Y.
Elless, M. P.
and
Foss, J. E.
2000.
Incorporation of Radioactive Contaminants into Pyroaurite-Like Phases by Electrochemical Synthesis.
Clays and Clay Minerals,
Vol. 48,
Issue. 2,
p.
266.
Loyaux-Lawniczak, Stéphanie
Refait, Philippe
Ehrhardt, Jean-Jacques
Lecomte, Paul
and
Génin, Jean-Marie R.
2000.
Trapping of Cr by Formation of Ferrihydrite during the Reduction of Chromate Ions by Fe(II)−Fe(III) Hydroxysalt Green Rusts.
Environmental Science & Technology,
Vol. 34,
Issue. 3,
p.
438.
Benali, Omar
Abdelmoula, Mustapha
Refait, Philippe
and
Génin, Jean-Marie Robert
2001.
Effect of orthophosphate on the oxidation products of Fe(II)-Fe(III) hydroxycarbonate: the transformation of green rust to ferrihydrite.
Geochimica et Cosmochimica Acta,
Vol. 65,
Issue. 11,
p.
1715.
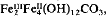 the Fe(II):Fe(III) ratio being independent of the Fe(II):Fe(III) ratio in the initial reaction mixture. It shows all the XRD peaks reported for this compound. Visible-near IR shows a broad peak at 650 nm which is ascribable to intervalence charge transfer and therefore the absorbance maximum decreases with increasing degree of oxidation. Wetting the material with compounds containing hydroxyl groups (such as glycerol or glucose) retards the oxidation of the otherwise very oxidation-sensitive compound. Similar polar organic compounds may stabilize the Fe(II)Fe(III) hydroxy-carbonate in wet soils. The importance of the green rust phase as an intermediate in the formation and transformation reactions of oxides and oxyhydroxides of iron in natural environments should not be ignored.
the Fe(II):Fe(III) ratio being independent of the Fe(II):Fe(III) ratio in the initial reaction mixture. It shows all the XRD peaks reported for this compound. Visible-near IR shows a broad peak at 650 nm which is ascribable to intervalence charge transfer and therefore the absorbance maximum decreases with increasing degree of oxidation. Wetting the material with compounds containing hydroxyl groups (such as glycerol or glucose) retards the oxidation of the otherwise very oxidation-sensitive compound. Similar polar organic compounds may stabilize the Fe(II)Fe(III) hydroxy-carbonate in wet soils. The importance of the green rust phase as an intermediate in the formation and transformation reactions of oxides and oxyhydroxides of iron in natural environments should not be ignored.



