Assessment of the adequacy of the left ventricle to support a biventricular circulation in newborns with left ventricular hypoplasia remains a critical issue in paediatric cardiology. If possible, biventricular repair is desirable as single ventricle palliation is associated with a reduced life expectancy and increased morbidity.Reference Feinstein, Benson and Dubin 1 The inappropriate selection of a biventricular repair in newborns whose left hearts are too small, restrictive, or obstructed results in left atrial hypertension with pulmonary vascular disease, right heart failure, and poor systemic perfusion, leading to poor outcomes.Reference Tchervenkov, Tahta, Jutras and Beland 2 Various echocardiographic measures are used to aid decision making regarding the correct surgical strategy,Reference Colan, McElhinney, Crawford, Keane and Lock 3 whereas cardiac MRI has been proposed as an adjunct to echocardiography to assess left ventricular size and output in these cases.Reference Grosse-Wortmann, Yun and Al-Radi 4 Phase-contrast MRI has also recently been used to measure the distribution of the foetal circulation in normal controls and late gestation foetuses with left-sided CHD.Reference Seed, van Amerom and Yoo 5 – Reference Prsa, Sun and van Amerom 7 In keeping with the flow theory of cardiac development, foetal MRI has demonstrated reduced oval foramen flow in the setting of diminished growth of left heart structuresReference Al Nafisi, van Amerom and Forsey 6 , Reference Rudolph 8 (Fig 1). On the basis of previous trials of maternal hyperoxygenation in normal foetuses and foetuses with CHD,Reference Kohl 9 – Reference Szwast, Tian, McCann, Donaghue and Rychik 11 we hypothesised that venous return to the underfilled left ventricle in this case of borderline left ventricular hypoplasia with atrial septal restriction could be augmented by inducing pulmonary vasodilatation through the administration of maternal oxygen.
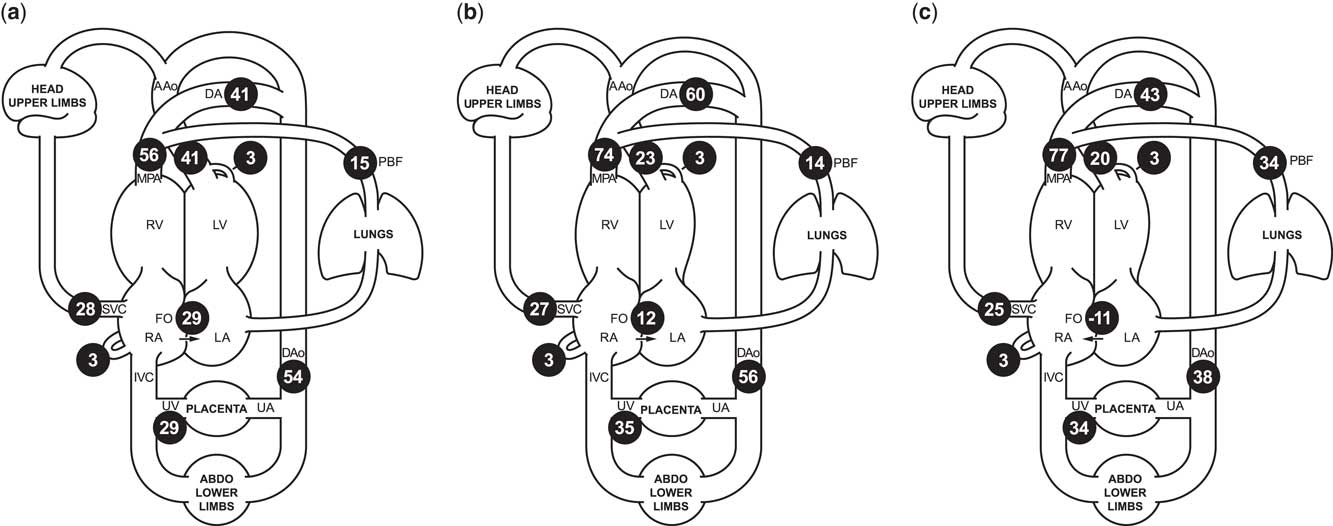
Figure 1 Cardiac MRI flow distribution in the foetal circulation, shown as mean percentages of the combined ventricular output (CVO) in ( a ) 40 normal late gestation foetuses, and ( b ) our case of borderline left ventricular hypoplasia, before maternal hyperoxygenation, and ( c ) following 20 minutes of maternal hyperoxygenation with 70% oxygen by non-rebreathing mask. Foramen ovale shunt is calculated as the difference between the ascending aorta and pulmonary blood flow. The reported mean percentages of the distribution of CVO required minimal adjustment to conform to a principle of conservation of flow across the foetal circulation, using a published model extrapolated from measured flows using constrained non-linear optimisationReference Prsa, Sun and van Amerom 7 . AAo/Dao=ascending/descending aorta; DA=ductus arteriosus; FO=foramen ovale; MPA=main pulmonary artery; PBF=pulmonary blood flow; RA/LA=right/left atrium; RV/LV=right/left ventricle; SVC/IVC=superior/inferior vena cava; UA/UV=umbilical artery/vein.
Case report
We present a case of borderline left ventricular hypoplasia diagnosed in foetal life. Foetal echocardiography showed significant ventricular disproportion, mitral valve diameter 6 SDs below the mean, and restrictive right-to-left flow at the oval foramen. The ascending aorta was small, and colour imaging revealed that a proportion of the blood supply to the upper body was being supplied by the right ventricle via the arterial duct, with retrograde flow across the aortic isthmus. Foetal cardiac MRI at 37 weeks’ gestation also showed ventricular disproportion (Supplementary Figures S1 and S2). Blood flow measurements made using cine phase-contrast imaging with metric optimised gatingReference Seed, van Amerom and Yoo 5 revealed a 50% reduction in ascending aortic flow compared with normal controls, with a 3:1 ratio of right ventricular to left ventricular output (normal 1.3:1). Pulmonary blood flow was normal. Oval foramen flow was significantly lower than the normal foetal mean (Fig 1), consistent with possible flow restriction across the atrial septum.
We hypothesised that maternal hyperoxygenation would induce pulmonary vasodilationReference Rudolph 8 and increase filling of the left ventricle. However, whereas maternal hyperoxygenation (20 minutes of 70% oxygen by non-rebreathing mask) caused a nearly twofold increase in pulmonary blood flow by MRI (Fig 1), the increased venous return did not augment the ascending aortic outflow. Instead, there was a reversal of atrial shunting, suggesting that valvar obstruction and ventricular hypoplasia were restricting flow across the left heart.
The child was born at term, with birthweight 2.89 kg, oxygen saturations 98–100% preductally and 78–84% postductally. Echocardiography demonstrated a nearly apex-forming left ventricle and small mitral (annulus 7.0 mm, z score −4.3) and aortic (annulus 4.8 mm, z score −4.6) valves. Prostaglandin E1 treatment was started to ensure systemic perfusion. Postnatal MRIs (Fig 2) showed an indexed left ventricular end-diastolic volume of 27 ml/m2 on the 1st day of life and 35 ml/m2 at 1 week, a gradual increase in pulmonary blood flow, yet no associated increase in left ventricular output. Notwithstanding, prostaglandin treatment was discontinued to assess whether a biventricular circulation was feasible following closure of the arterial duct, although this resulted in poor systemic perfusion with metabolic deterioration. At 9 days of life, the child underwent a hybrid procedure, consisting of bilateral pulmonary artery banding and ductal stenting.
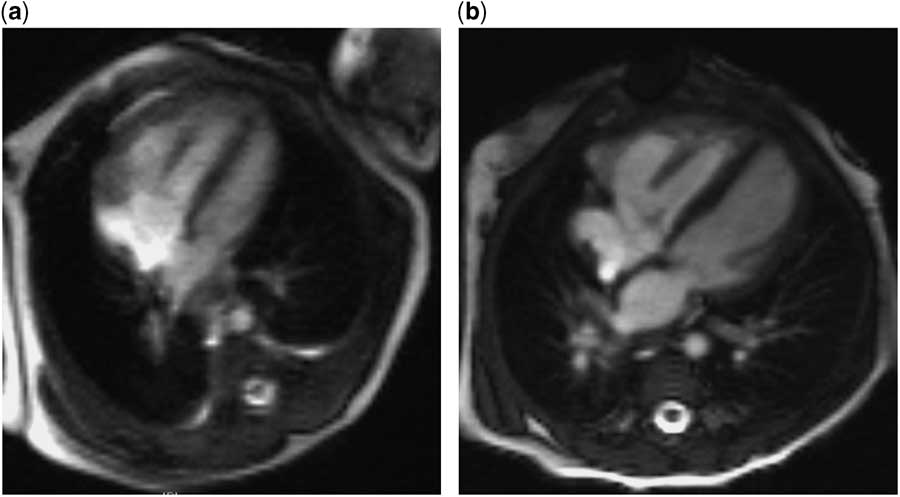
Figure 2 Postnatal cardiac MRI images of the four-chamber view. ( a ) At day 1 of life, the left ventricle appears small and has an indexed left ventricular end-diastolic volume (LVEDVi) of 27 ml/m2, and measured ascending aortic flow is 1.57 L/minute/m2. ( b ) At 4.5 months of life, subsequent to a neonatal hybrid procedure of bilateral pulmonary artery banding and arterial ductal stenting, the left ventricular size appears normal, with an LVEDVi of 62 ml/m2, and ascending aortic flow is 3.14 L/minute/m2.
Cardiac MRI at 4 months of age demonstrated further growth of the left ventricle, with a left ventricular end-diastolic volume index of 62 ml/m2, and ascending aortic flow in excess of 3 L/minute/m2. Echocardiography showed no inflow or outflow obstruction and mitral valve diameter 2.8 SDs below the mean. In view of the encouraging investigations, the child underwent elective biventricular repair at 5 months of age, including arch reconstruction, duct ligation, and atrial septal defect creation. She made an uneventful recovery and is clinically well, gaining weight appropriately, on no cardiac medications 18 months later. She has mild residual mitral and aortic valvar stenosis, but there is no evidence of pulmonary hypertension.
Discussion
The pathogenesis of hypoplastic left heart syndrome, ranging from the borderline left ventricle to complete aortic and mitral atresia, is not fully clear. It is likely multi-factorial, relating to genetic and anatomic factors, with restricted flow across the foetal oval foramen in the setting of low pulmonary blood flow, also impairing the development of the left-sided structures.Reference Feinstein, Benson and Dubin 1 The possibility of finding an option to aid the antenatal development of the borderline ventricle is extremely engaging. Furthermore, decision making regarding the adequacy of the borderline left ventricle for a biventricular repair is complex, and an improved understanding of the physiology and natural history of patients with this spectrum of abnormalities is desirable. Pulmonary vasodilation in response to maternal hyperoxygenation has been described in foetal sheepReference Rudolph 8 and shown in human foetuses by echocardiography.Reference Kohl 9 – Reference Szwast, Tian, McCann, Donaghue and Rychik 11 As far as we are aware, this account represents the first report of an increase in pulmonary blood flow in response to maternal hyperoxygenation by foetal cardiac MRI.
Thomas Kohl has proposed maternal hyperoxygenation as a treatment for achieving growth of underdeveloped left heart structuresReference Kohl 9 and reported encouraging results in 13 late gestation foetuses, although with no control group. Importantly, oxygenation did not cause ill effects to the foetuses such as ductal constriction or cardiac dysfunction, or mothers, for example, pulmonary oedema, in keeping with previous evidence of the acute safety of maternal hyperoxygenationReference Szwast, Tian, McCann, Donaghue and Rychik 11 and reversibility of its effects on foetal circulation.Reference Rasanen, Wood, Debbs, Cohen, Weiner and Huhta 10 The safety of chronic maternal hyperoxygenation in the setting of intrauterine growth restriction has been addressed by a Cochrane Review,Reference Say, Gülmezoglu and Hofmeyr 12 which concluded that, although three randomised controlled trials showed no evidence of adverse effects from maternal oxygen therapy, there was a potential for harm from maternal oxygen therapy resulting from reduced placental blood flow based on animal models.
In our case, maternal hyperoxygenation led to a clear increase in foetal pulmonary blood flow, but no increase in left ventricular output. These findings suggest impaired left ventricular filling owing to restrictive left ventricular physiology and/or anatomical obstruction. However, it is interesting to consider whether a more prolonged exposure to maternal oxygenation could have improved left heart growth in utero, potentially allowing a biventricular repair in the neonatal period, just as improved left ventricular filling via the pulmonary circulation in this patient and others reported in the literature resulted in the growth of left heart structures after birth.Reference Ballard, Tibby and Miller 13
Financial Suppor t
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Conflicts of Interest
None.
Ethical Standards
The authors assert that all work reported complies with the ethical standards of the Helsinki convention, and consent for publication has been granted by the patient’s family.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S1047951114001802






