Background
Owing to the centralisation of services such as cardiology, cardiac surgery, and intensive care in many parts of the world, children with critical cardiac disease presenting to local hospitals frequently require stabilisation and safe transport to specialist centres for diagnostic, surgical, and/or critical care intervention.Reference Ramnarayan, Thiru and Parslow 1 – Reference Lundstrom, Berggren and Bjorkhem 3 The inter-hospital transport of this group of patients can present particular clinical and logistical challenges.Reference Orr and Kuch 4 Children with critical cardiac disease often have greater severity of illness and require significant stabilisation before transport; their complex circulatory physiology, particularly in children who have had multiple previous cardiac interventions, is more likely to be affected by events common during transport such as acceleration–deceleration and changes in ventilation; the potential for rapid physiological deterioration means that close monitoring by experienced staff is a crucial consideration; and the absence of a clear cardiac diagnosis in many cases complicates clinical decision-making and the selection of an appropriate destination unit. Inter-hospital transport of children with critical cardiac disease should usually be undertaken by a team with specialist training and experience in the areas of cardiac critical care and transport.Reference Orr, Felmet and Han 5
In this expert review, we will cover aspects related to the transport of critically ill children with cardiac disease such as referral triage and advice, transport decision-making, aeromedical and international transport, and the management of specific cardiac conditions during transport.
Referral triage and advice
The main purpose of triage before transport is to assess the clinical condition of the patient, including whether a cardiac diagnosis is confirmed and how critically ill the patient is; assess the local capabilities and resources available – for example, availability of staff to perform critical interventions and diagnostic tests, and whether telemedicine is available and appropriate; and provide recommendations to stabilise the patient while waiting for a transport team to arrive.Reference Warren, Fromm and Orr 6 Advice given should be tailored to the clinical scenario and the capabilities of the referring centre. At the same time, there must be a recognition that some urgent interventions cannot wait until the arrival of the transport team. Empowering local staff to use available resources effectively is an important part of the triage process.
Clinical assessment and advice
Clinical assessment and advice provision should be completed rapidly and, if necessary, in consultation with relevant specialists (cardiologist, cardiac intensivist). A systematic and structured approach is important. Key points relating to clinical assessment and advice are shown in Table 1.
Table 1 Key points relating to clinical assessment and advice during triage for transport.
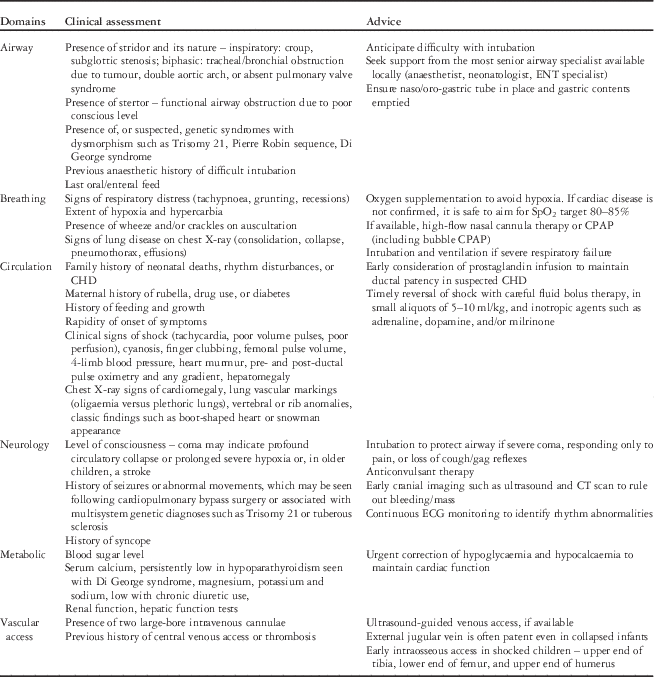
CPAP=continuous positive airway pressure; ENT=ear, nose and throat.
Assessment and optimisation of local resources
Children with critical cardiac disease are often severely ill and require the most experienced staff available to care for them. A team-based approach should be adopted. 7 Staff with airway skills and those with knowledge related to the cardiac condition should be available. While in some settings this might be the cardiac intensive care team, in many settings members of anaesthetic and paediatric teams should be present during stabilisation to complement each other’s skills and knowledge. Neonatologists should be involved in acute care of neonates where possible. In settings in which local staff resources are limited, the most experienced person (or people) with advanced life support skills should lead the clinical team.
The unavailability of equipment and trained operators may limit the capability of local staff. Staff with even basic echocardiographic skills are likely to be unavailable in many settings, highlighting the importance of clinical assessment in cardiac disease. Anaesthetic staff may be unfamiliar with intubation in children or lack advanced airway equipment – supraglottic airway devices can provide a safe alternative option to manage the airway and may provide effective ventilation in such cases.Reference Ostermayer and Gausche-Hill 8 Intraosseous access can serve as an effective alternative to central venous access even when staff have limited experience in obtaining venous access.Reference Fiorito, Mirza and Doran 9
Ongoing advice from the remote specialist centre (or transport team) is important to support the local hospital during stabilisation. Traditional telephone-based approaches may be replaced by video consultation models in the future.Reference Satou, Rheuban and Alverson 10 Advances in technology mean that even mobile phones can be used for this purpose. Although the existing evidence base is weak, adoption of telemedicine has been shown to improve the quality of advice provided during paediatric critical care referral.Reference Dayal, Hojman and Kissee 11
Transport
General principles
The general principles related to the transport of the child with critical cardiac disease are as follows:
-
∙ As interventions while travelling in the ambulance or aircraft can be technically difficult and associated with a higher risk of complications, stabilisation is best performed at the referring hospital before transport.
-
∙ Achieving physiological stability may not be possible in some scenarios where a definitive intervention can only be performed at the specialist centre. Typical examples are transposition of the great arteries with poor mixing – where an urgent balloon atrial septostomy is required – and obstructed total anomalous pulmonary venous return – where emergency surgery is required. Prior awareness of these conditions should highlight to the transport team that once life-saving interventions such as intubation and ventilation have been performed, the child should be rapidly transferred to the specialist centre. When there is no clear diagnosis, failure to respond to key interventions as expected – for example, refractory severe hypoxia despite adequate ventilation – should warn the transport team that a “time-critical” transfer is required. In rare circumstances in which “time-critical” transport involves very long distances, it may be necessary to stabilise the child before transport by placing them on extracorporeal life support for the transport.Reference Perez, Butt and Millar 12
Transport logistics
Type of team
The risk of complications during transport and a strong evidence-base supporting the use of specialist retrieval teams to perform critical care transports suggest that most children with cardiac disease should be transported by specialist transport teams.Reference Ramnarayan, Thiru and Parslow 1 , Reference Orr, Felmet and Han 5 Specialist teams are also more likely to maintain up-to-date skills by regular exposure in clinical practice and during simulated training. Rarely, it may be necessary to transport patients using local staff, mainly owing to lack of timely availability of a specialist team or for time-critical transfers; in these cases, staff with the most experience in managing patient deterioration during transport should accompany the patient. These teams may sometimes be local adult or neonatal critical care transport teams.
Transport equipment
The risk of complications during transport means that children with cardiac disease being transported to a specialist centre should undergo multi-parameter monitoring – continuous echocardiogram and heart rate, oxygen saturations, non-invasive or invasive blood pressure measurement, temperature, and end-tidal CO2 for ventilated patients – using portable, battery-operated monitors.Reference Sarfatti and Ramnarayan 13 The team should have access to a portable mechanical ventilator that is battery-operated, or pneumatically driven, and can ventilate patients of various ages – newborn through adolescent. A defibrillator that can be operated in manual mode – ideally combined with transthoracic pacing capabilities – is recommended for most patients. A standardised bag with transport supplies to manage the airway, breathing, and circulation should be available.Reference Dawes, Ramnarayan and Lutman 14
Aeromedical transport
The primary motivation for air transport is to achieve a speedy patient transfer to the specialist centre. However, several factors, such as how quickly a helicopter or fixed-wing aircraft is available, how far the landing sites are from both hospitals, and weather considerations, affect any expected time-savings from aeromedical transport. On the other hand, air transport carries small but significant risks to patients and staff. Therefore, the decision to fly must be made after careful consideration of the risks and the expected benefits in each case.
The main patient risks from aeromedical transport relate to physiological changes associated with altitude and the G-forces associated with flight.Reference Valente, Sherif and Azen 15 , Reference Farmer 16 With increasing altitude, gas expansion can pose a significant problem when it is trapped in a body cavity – for example, pneumothorax, pneumoperitoneum, gastric cavity – or contained within medical equipment – for example, endotracheal tube cuff and urinary catheter cuff. Attention should be paid to draining trapped gases before flying – for example, nasogastric tube on free drainage and chest drain inserted – and to deflate/inflate cuffs during take-off and landing. Hypoxia from flying at an altitude can be dangerous in cardiac disease with pre-existent cyanosis – supplemental oxygen may be required. Take-off and landing are particularly dangerous in shock states because of G-forces; therefore, head down or feet up during take-off to prevent pooling of blood in the lower extremities, or the use of fluid boluses to increase preload, may be needed.
Noise and vibration associated with helicopter transport, and the cramped working environment, impose an additional burden on the staff caring for a critically ill child. Ear plugs for children and headsets/helmets for staff should be used to minimise prolonged exposure to noise.
International transports
The main feature of international transports is the long distance involved. For high acuity patients, a dedicated air ambulance may be the most effective means of transport, providing adequate physical space and access to oxygen and/or power; however, in most other cases, a commercial aircraft is preferred owing to speed and the advantage of avoiding multiple refuelling stops. Military aircrafts are also frequently used for international transports. Primary considerations related to such transports are staff numbers and expertise – for example, for long transports stretching over several hours, more than two members of staff may be required to avoid fatigue, and where patient deterioration is expected, staff should be able to provide critical care interventions in flight – and equipment – for example, battery-powered with additional batteries available, ventilators with a compressor-driven turbine to avoid the need for heavy air cylinders and able to function using low-flow oxygen, and ability of equipment to be safely secured in the aircraft.Reference Beninati and Jones 17
Transports in resource-limited settings
The limited healthcare infrastructure in low- and middle-income countries poses several different challenges relating to the transport of children with critical cardiac disease.Reference Duke 18 Many low- and middle-income countries’ settings do not have established services for cardiology, cardiac surgery, or paediatric intensive care. As antenatal echocardiography is unavailable, the majority of infants with cardiac disease are born in village health outposts and smaller town health centres/hospitals or at home, and are diagnosed in the postnatal period or later. Vital medications such as prostaglandin may be unavailable, and there is a scarcity of dedicated staff trained in transport. Depending on the complexity of the lesion and the resources available locally, patients may be managed locally awaiting surgical input, by visiting cardiac charity missions such as Chain of Hope, United Kingdom, or International Children’s Heart Foundation, United States of America; transported to a larger regional centre where corrective or palliative surgery may be possible; or require international transport to a high-income country for surgical intervention.
The key considerations for transport are the clinical status of the patient, the end point of management, which teams need to be involved in the discussions/ management plan, the distance required to travel, who will accompany the patient on the transfer, what resources are available for the transfer – equipment, mode, and vehicle used – and the discussions with the family/carers/patient. It may be necessary to adapt existing adult ambulances to secure the child using a bassinet, kangaroo pouch, or car seats (Fig 1). There must be a clear plan to ensure continuous monitoring, whether this is direct visualisation or use of limited resources, for example, oximetry. The need for venous access should be assessed, and a decision should be made regarding whether the patient should be fed during the transfer. The practicalities of storage of medications, labelling of drugs, and delivery of intravenous medications under clean conditions should be considered. For long-distance international transports in commercial aircraft, a “grab bag” (limited, essential equipment) should be readily available, and secure venous access should be in place to deliver life-saving medications such as prostaglandin. Most commercial airlines would not be able to deliver oxygen at take-off and landing, and it may be necessary to carry portable oxygen cylinders or oxygen concentrators. Secure fixation of equipment to avoid dislodgement during turbulence is an important safety consideration. International volunteer transport teams should be aware of the medicolegal ramifications and ensure that their medical protection unions will cover such activity, keep detailed medical records, and obtain consent from the parents/carers and referring team before undertaking these transfers.
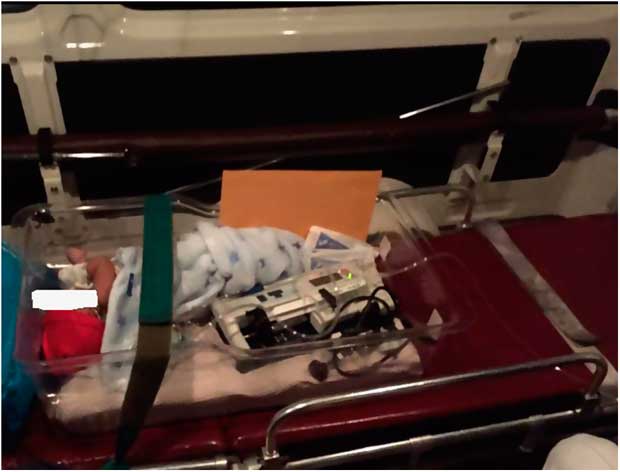
Figure 1 Transporting a neonate with transposition of great arteries in a low-middle income country from hospital to airport for a commercial flight. Note the use of an adult ambulance and the adaptation of a bassinet to secure the child.
Clinical management during transport
Key points related to clinical management by the transport team are summarised in Table 2.
Table 2 Key messages for the clinical assessment and stabilisation of children with critical cardiac disease.
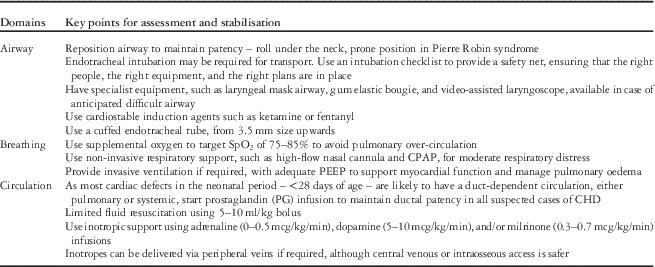
CPAP=continuous positive airway pressure; ECG=echocardiogram; PEEP=positive end-expiratory pressure.
Management of specific cardiac conditions during transport
Specific cardiac conditions are grouped into six clinically relevant categories and key points relating to their management during transport are summarised below.
Antenatally diagnosed CHD
Prostaglandin infusion at small doses (5–10 ng/kg/minute) should be used to maintain ductal patency during the transport of the baby to the specialist centre. Intubation is not usually routinely required if the dose does not exceed 15 ng/kg/minute.Reference Meckler and Lowe 19 , Reference Browning Carmo, Barr and West 20
The “blue neonate”
If the diagnosis is in doubt, a hyperoxia test – SpO2 or PaO2 response to short-term administration of 100% oxygen – may be helpful to differentiate lung disease from cardiac disease. In most cases of cyanosis where a cardiac cause is suspected, ductal patency should be maintained using a prostaglandin infusion. Intubation is usually required when the dose of prostaglandin infusion is greater than 15 ng/kg/minute owing to the risk of apnoea.
d-transposition of great arteries
High doses of prostaglandin may be required to reopen a closed duct (10–20 ng/kg/minute, 20–50 ng/kg/minute in some cases). Failure to improve oxygen saturations with a prostaglandin infusion, particularly in a neonate with cyanosis and plethoric lungs, indicates that an urgent balloon atrial septostomy is required, usually under specialist cardiology care. Time to septostomy can be an important factor in the survival and neurodevelopmental outcomes of newborns with d-transposition of the great arteries; therefore, rapid transport is essential. Fluid administration and adrenaline (epinephrine) infusions may be useful but should not delay transport.
Obstructed total anomalous pulmonary venous return
Urgent transfer for surgery may be required if profound cyanosis unresponsive to conventional therapies – prostaglandin infusion and ventilation – suggests a diagnosis of total anomalous pulmonary venous return. The presence of systemic hypoperfusion with lactic acidosis makes the diagnosis of obstructed very likely, but without echocardiography obstructed total anomalous pulmonary venous return is difficult to distinguish from persistent pulmonary hypertension of the newborn, and a short trial of inhaled nitric oxide may be required. Lack of response should lead to termination of the trial of inhaled nitric oxide to avoid further pulmonary vasodilatation; prostaglandin infusion may not always be helpful, but it should always be started to assess response.
The “shocked” neonate
Septic shock and obstruction to systemic cardiac output are the main differential diagnoses to consider in the “shocked” neonate. In most cases of neonatal shock where a cardiac cause is suspected, ductal patency should be maintained using a prostaglandin infusion. Intubation is usually required when the dose of prostaglandin infusion is >15 ng/kg/minute or evidence of systemic hypoperfusion is present. Pulmonary over-circulation should be prevented by maintaining a pulmonary to systemic perfusion (Qp:Qs) ratio of 1:1, usually achieved by adjusting the FiO2 to target oxygen saturations of 75–85% and normalising the pH and pCO2. Severe acidosis can be treated with sodium bicarbonate to improve myocardial function; intravenous inotropic support can be provided by dopamine and/or adrenaline infusions; and milrinone may be useful to provide lusitropy, inotropy, and to reduce afterload. Blood transfusion to optimise oxygen-carrying capacity and measures to decrease oxygen consumption, such as sedation, muscle relaxation, and avoidance of hyperthermia, should also be considered. In hypoplastic left heart syndrome with a restrictive atrial connection, shock does not respond to conventional management, indicating a specific situation in which a “time-critical” transfer to the specialist centre will be required.
The “blue” infant or child
Cyanosis may be a presenting feature in a child in the setting of undiagnosed cyanotic heart disease, repaired heart defects, or from pulmonary pathology. The management of spells during transport in cases of tetralogy of Fallot involves the administration of 100% oxygen, intravenous morphine, β-blockers such as propranolol, fluid bolus to increase preload, and increasing the systemic vascular resistance by vasoconstrictors such as phenylephrine, metaraminol, or noradrenaline infusion. Intubation and ventilation may be required when response to conventional treatment is inadequate and/or if repeated spells occur in a short period of time, with associated metabolic acidosis. In cases of blocked systemic-pulmonary shunt, fluid volume administration and intravenous heparin are indicated, with rapid transfer to the specialist centre for urgent intervention.
The infant or child in heart failure
Main treatment principles include oxygen therapy, judicious fluid management to avoid fluid overload, diuretic therapy, and support for the failing myocardium. Myocardial support can be provided by positive pressure ventilation, either using non-invasive ventilation or intubation and ventilation. Support for the failing myocardium can be provided by the use of inotrope infusions (adrenaline, dopamine, dobutamine, or milrinone).
The child with an abnormal heart rhythm
Minimum monitoring during transport should include continuous electrocardiogram, pulse oximetry, regular blood pressure, and respiratory rate, and defibrillation pads should be in place on the child during the transport in preparation for intervention. A defibrillator capable of delivering synchronous and asynchronous direct current (DC) shocks, as well as transthoracic pacing, should be readily available.
Supraventricular tachycardia
If adenosine fails to restore sinus rhythm and shock persists, or if intravenous access is not available, synchronised cardioversion (1 J/kg) should be administered. Cardioversion will usually require an infant’s airway to be stabilised by intubation first, although older children can be managed with sedation alone – using midazolam or ketamine. An infusion of amiodarone, at a loading dose of 25 μg /kg/minute for 4 hours, and then 5–10 μg/kg/minute, should be considered in refractory cases.
Ventricular tachycardia
Haemodynamic instability should be managed with DC cardioversion (1–2 J/kg) once the airway and breathing have been stabilised. Polymorphic ventricular tachycardia – torsades de pointes – can be treated with intravenous magnesium sulphate (25 mg/kg).
Complete heart block
Stabilisation and initiation of agents such as isoprenaline or adrenaline infusions may be used to increase the heart rate. In refractory complete heart block, transcutaneous pacing may be required during transport.
Conclusion
With increasing focus on the centralisation of specialist services in fewer centres, or lack of specialist services in many parts of the world, the need for inter-hospital transport of children with critical cardiac disease is only likely to rise. The key principles of safe transport include robust triage and advice provision, clinical stabilisation before transport, and anticipation of potential complications during transport.
Acknowledgements
None.
Financial Support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Conflicts of Interest
None.







