Anatomically corrected malposition of the great arteries is a rare and very interesting cardiac defect with peculiar alignment of the great arteries. Reference Van Praagh, Durnin and Jockin1–Reference Bernasconi, Cavalle-Garrido, Perrin and Anderson10 Initially named anatomically corrected “transposition” of the great arteries, Reference Van Praagh and Van Praagh5 this malformation stimulated an interesting debate on morphology and nomenclature of CHD. Reference Van Praagh, Durnin and Jockin1–Reference Arciprete, Macartney, de Leval and Stark9 The ventriculoarterial connections are concordant, with the pulmonary artery usually posterior and aligned with the morphologically right ventricle, while the aorta arises from the morphologically left ventricle but usually located anteriorly and with a quite variable infundibular morphology. Reference Van Praagh, Durnin and Jockin1–Reference Thiene, Pellegrino, Maddalena and Gallucci6 Notably, in this cardiac malformation, the great arteries are parallel to each other rather than in a spiral relationship.
This CHD has been described in all types of viscero-atrial arrangements, with and without atrioventricular concordance, with sub-aortic, sub-pulmonary, or bilateral infundibula and associated with many other cardiac malformations, such as tricuspid atresia and coronary artery abnormalities. Reference Van Praagh, Durnin and Jockin1–Reference Arciprete, Macartney, de Leval and Stark9 The difference between so-called isolated ventricular inversion and “corrected malposition of the great arteries” is that in ventricular inversion the great arteries maintain their normal spiral relashionship. Reference Van Praagh, Durnin and Jockin1–Reference Thiene, Pellegrino, Maddalena and Gallucci6
In patients with viscero-atrial situs solitus, two anatomical forms of anatomically corrected malposition of the great arteries were reported. The more frequent type consists in atrioventricular concordance (d-ventricular loop) with the aorta arising anteriorly and to the left from the left-sided morphologically left ventricle [S,D,L] with physiological normal blood circulations. Reference Van Praagh, Durnin and Jockin1,Reference Kirklin, Pacifico, Bargeron and Soto3–Reference Thiene, Pellegrino, Maddalena and Gallucci6 The more rare type consists in atrioventricular discordance (l-ventricular loop) with the aorta arising anteriorly and to the right from the right-sided morphologically left ventricle [S,L,D]. Reference Pasquini, Sanders and Parness2,Reference Van Praagh and Van Praagh5–Reference Arciprete, Macartney, de Leval and Stark9 In this latter form, the blood circulations are parallel to each other and the haemodynamic status is that of transposition of the great arteries.
We report a child with anatomically corrected malposition of the great arteries [S,L,D], successfully treated with Mustard operation, associated with a mutation of Nodal gene.
Case report
A full-term female neonate with a prenatal diagnosis of “ventricular inversion” was admitted, at birth, to the neonatal ICU with profound cyanosis. Two-dimensional colour Doppler echocardiography showed levocardia, situs solitus of the atria, and discordant atrioventricular connections with l-loop of the ventricle (Fig 1a and c). The ventriculoarterial connections were concordant with the aorta arising anteriorly and right-sided from the right-sided morphologically left ventricle (Figs 1b, 2a and b). The pulmonary artery arose posteriorly and left-sided from the left-sided morphologically right ventricle (Fig 2a). The two great arteries are parallel to each other without spiralisation (Fig 2b). There was a small interatrial communication that required a Rashkind procedure at 3 days of age. The ventricular septum was intact (Figs 1a and 2a). The diagnosis of anatomically corrected malposition of the great arteries [S,L,D] was done, and the patient underwent a successful Mustard operation at 5 months of age. No extracardiac anomalies were detected clinically and by cerebral and abdominal ultrasound examinations.
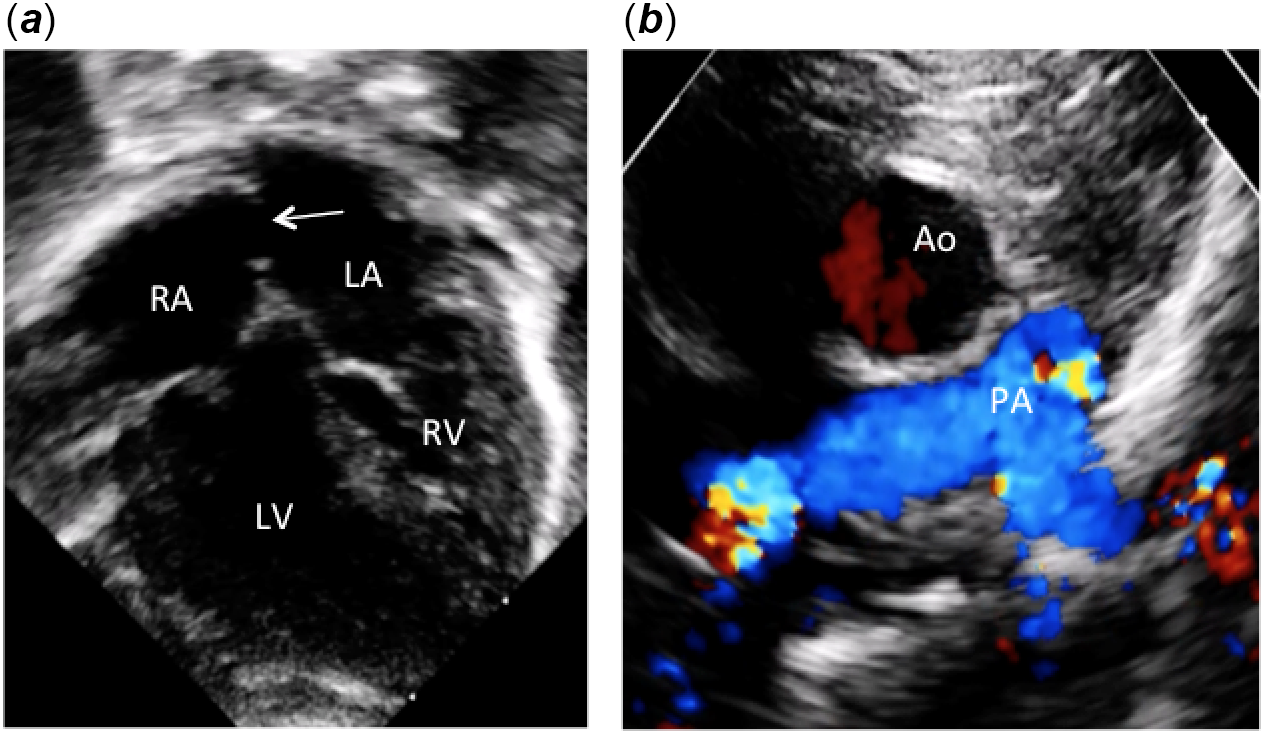
Figure 1. Apical and parasternal two-dimensional echocardiographic pictures. (a) Apical four-chamber view showing atrioventricular discordance with l-ventricular loop. Note the inverted atrioventricular valve offsetting and the large atrial septal communication (arrow) after Rashkind procedure. (b) Parasternal short axis view showing the aorta located anteriorly and to the right of the pulmonary artery. Ao: aorta; PA: pulmonary artery; LV: left ventricle; RV: right ventricle; RA: right atrium; LA: left atrium.
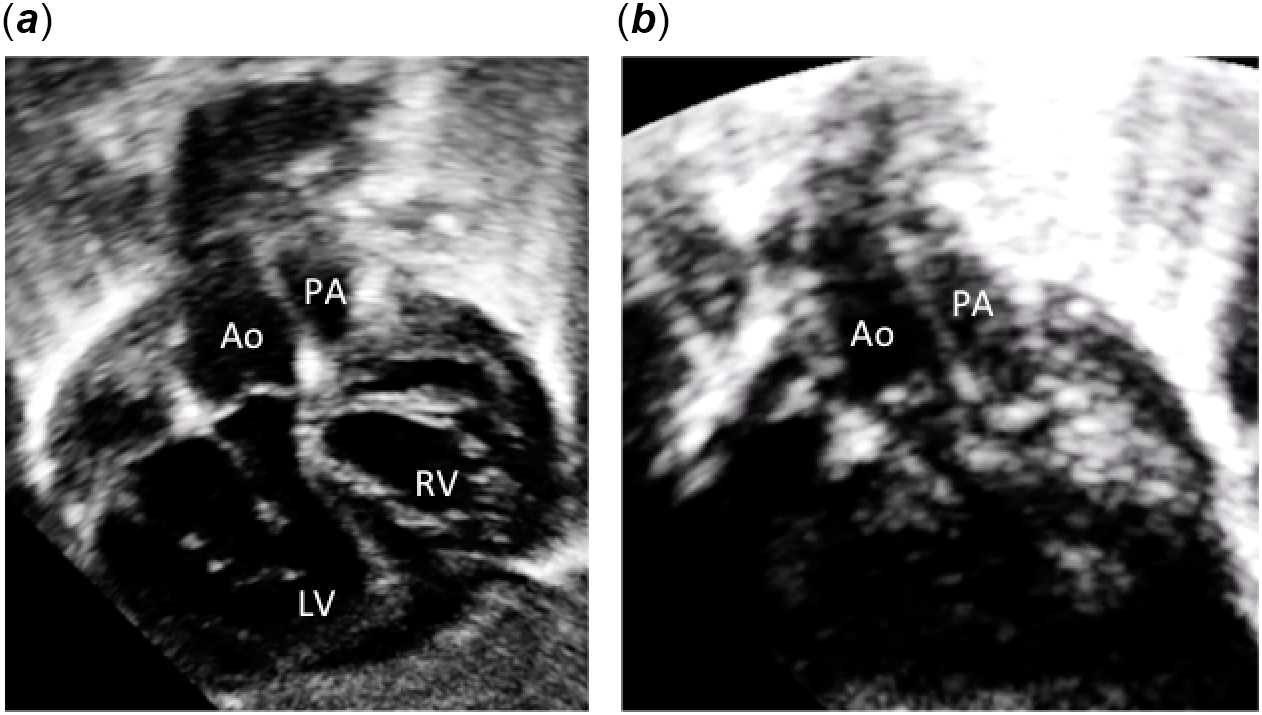
Figure 2. Subcostal two-dimensional echocardiographic pictures. (a) Subcostal view showing ventriculo-arterial concordance. The aorta arises anteriorly from the right-sided morphologically left ventricle and the pulmonary artery arises posteriorly from the left-sided morphologically right ventricle. (b) Subcostal view showing the short sub-aortic and sub-pulmonary infundibulum and the parallel position of the great arteries. Ao: aorta; PA: pulmonary artery; LV: left ventricle; RV: right ventricle; RA: right atrium; LA: left atrium.
Based on the rarity and complexity of the cardiac anatomy, a genetic study was suggested. Next Generation Genetics testing was performed by the Twist Custom Panel kit (clinical exome – Twist Bioscene) and analysed on NovaSeq6000 (Illumina) platform. The heterozygous c.817G > A missense variant in Nodal gene was detected, leading to the aminoacidic change p. Ala273Thr (rs770958126).
The genetic variant was validated on re-extracted DNA and verified by bidirectional Sanger sequencing. Standard chromosomal analysis and SNParray were normal.
Discussion
Nodal gene is a member of the TGF-β superfamily of developmental regulators and is critical for early embryonic patterning in vertebrates. Nodal signalling has been shown to play an important role in anterior–posterior axis patterning and left–right axis determination. Reference Brennan, Norris and Robertson11 Disruption of Nodal signalling in mammals has been linked to cardiac malformations due to defects in left–right patterning of the embryo, such as heterotaxia with left–right asymmetry of the thoracoabdominal organs. Reference Mohapatra, Casey and Li12 Loss-of-function mutations in several genes belonging to the Nodal signalling pathways, including Nodal itself, Cripto, Cryptic, Lefty1, Lefty2, and FoxH1, have been identified in patients with cardiac laterality defects. Reference Roessler, Pei and Ouspenskaia13 Interestingly, mutations in Nodal gene in humans have been detected also in cases of isolated or syndromic transposition of the great arteries. Reference Mohapatra, Casey and Li12 It is possible that the same genetic defect could lead to a variety of cardiac laterality malformations, ranging from heterotaxia to transposition of the great arteries with viscero-atrial situs solitus. Reference Versacci, Pugnaloni and Digilio14 In addition, epidemiological studies are showing familial aggregation of heterotaxia, d-transposition of the great arteries, and congenitally corrected transposition of the great arteries with situs solitus. In some of these families, multiple mutations in laterality genes, including Nodal and ZIC3, have been identified. Reference De Luca, Sarkozy and Consoli15
The present case is the first in which a mutation of Nodal gene is reported in association with anatomically corrected malposition of the great arteries with discordant atrioventricular and concordant ventriculoarterial connections [S,D,L]. Although there is no definitive evidence that the mutation reported is causal of this cardiac defect, we suggest that this genetic variant of Nodal gene could be considered pathogenetic in this peculiar case. Moreover, we do not know if this mutation could be the cause of the l-loop of the ventricle Reference Noël, Verhoeven and Lagendijk16 or of the specific conotruncal anomaly or both. However, the involvement of Nodal, a very important gene for the development of the left–right asymmetry, is not surprising in this complex cardiac malformation that shows evident signs of ventricles and great arteries laterality defects.
Acknowledgements
None.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflicts of interest
None.
Ethical standards
None.







