Introduction
The goal of effective, timely neuroprotection in acute human ischemic stroke remains elusive. Despite decades of preclinical, translational, and clinical investigation with innumerable agents and protocols, no strategy has yet proven to be beneficial. The need remains urgent, as even with current endovascular therapy (EVT) and impressive recanalization rates, only 46%−60% of patients, depending on selection criteria, are functionally independent by mRS at 90 days Reference Goyal, Menon and van Zwam1,Reference Hill, Goyal and Menon2 and only 10% are neurologically normal. Reference Goyal, Menon and van Zwam1 EVT affords new opportunities by combining neuroprotection with revascularization.
Neurotoxic Cascade
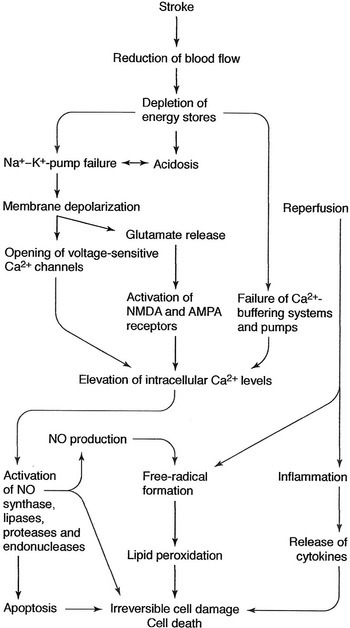
The neurotoxic cascade in acute ischemic stroke is summarized in Figure 1. Briefly, reduced blood flow leads to decreased adenosine 5-triphosphate generation, neuronal and Na/K depolarization, calcium influx, activation of apoptosis pathways, and cell death. Glutamate, the primary excitatory neurotransmitter, accumulates in the extracellular space, causing activation of N-methyl-D-aspartate (NMDA) and other receptors mediating calcium influx. Oxidative stress occurs from free radical generation, nitrosative stress from elevated calcium levels. Microglia and astrocytes produce inflammatory cytokines, toxic metabolites, and enzymes. Endothelial damage disrupts the blood brain barrier, increasing the inflammatory response of leukocytes and PMNs. T-cell involvement and cerebral edema further impair recovery. Ischemic injury to pericytes causes constriction of vascular channels to the neurovascular unit (NVU) reducing effective microvascular reperfusion. Reference Karsy, Brock, Guan, Taussky, Kalani and Park3

Figure 1: The ischemic cascade in acute stroke.
Historical Aspects
The oldest neuroprotective agent is therapeutic hypothermia (TH). It was first described in an Egyptian papyrus from 5000 years ago. It was used by Hippocrates to reduce hemorrhage in wounded soldiers and by Napoleon’s surgeons to reduce the pain of amputation. Thomas Willis observed its Lazarus-like restorative powers in the 17th century. An Oxford serving girl, hanged for bearing a child out of wedlock, was unexpectedly resuscitated hours later, prior to dissection in his lodgings, likely saved by a combination of hypothermia and hypoglycemia. TH was rediscovered in the 1930s for analgesia, and in the 1950s for neuroprotection during cardiac and neurological surgery. Reference Bigelow, Lindsay and Greenwood4,Reference Botterell, Lougheed, Scott and Vandewater5
The modern history of neuroprotection is based on cellular aspects of neuronal injury. Spielmeyer in 1925 Reference Spielmayer6 was the first to describe selective vulnerability of hippocampal CA-1 cells following transient global ischemia, confirmed by Russian physiologists in the 1950s. Global ischemic models were developed in the 1970s and 1980s and demonstrated the selective loss of specific neurons in the cortex, striatum, and hippocampus, initially in small animals and subsequently observed in postmortem studies in humans. Reference Pulsinelli, Brierly and Plum7 Animal models of focal ischemia, particularly in nonhuman primates, mimicking the human thromboembolic condition, were developed in the 1970s and 1980s. Reference Symon, Branston, Strong and Hope8 These studies led to the concept of a core and reversible ischemic penumbra in the 1970s. Reference Hakim9 Glutamate excitotoxicity leading to calcium influx was described in the 1980s Reference Rothman and Olney10 leading to trials of NMDA and AMPA antagonists for neuroprotection. Apoptotic neuronal cell death was targeted with caspase inhibition, mitochondrial pore inhibitors and rapamycin. Neuroinflammation was targeted in the 1990s and free radical scavengers such as PBN and NXY-059 were promising enough in preclinical trials Reference Siesjo, Agardh and Bengtsson11 in the 1990s to generate the clinical SAINT trials of the early 2000s. The “lazaroids” tirilizad and edaravone also progressed to extensive clinical trials. Cellular and vascular interventions to improve blood flow and metabolism included albumin Reference Belayev, Liu, Zhao, Busto and Ginsberg12 and nimodipine. Despite promising preclinical results using all these agents, translation to clinical efficacy has been consistently unsuccessful. Whole body and selective TH have been utilized since the 1950s for neuroprotection, with impressive preclinical results but physiological and logistical challenges in acute stroke continue to prevent their clinical application.
The challenges and failures of clinical translation led to the establishment of specific guidelines for preclinical studies by the Stroke Treatment Academic Industry Roundtable collaborators Reference Savitz, Baron and Fisher13 to standardize research protocols and maximize the potential for clinical application.
PreClinical Translation Failure
Between 1998 and 2016, over 12,000 acute stroke patients were enrolled in trials targeting free radicals, ion channels, excitotoxicity, immune modulation and inflammation, all of which failed or at best showed marginal benefit. Reference Karsy, Brock, Guan, Taussky, Kalani and Park3,Reference Rajah and Ding14 Notable examples of promising preclinical results with subsequent failed translation include: magnesium (NMDA ion channel inhibitor and calcium antagonist); selfotel, a potent NMDA antagonist which showed promise as a neuroprotectant in Phase 1 and 2 trials and revealed neurotoxic effects and increased mortality in 2 Phase 3 trials Reference Davis, Lees and Albers15 ; verapamil and nimodipine (calcium channel blockers); uric acid (free radical scavenger); edaravone and NXY-059 (antioxidants); activated protein C (multiple targets); statins (antioxidant, angiogenesis, antiinflammatory); UK-279,2767 neutrophil inhibitory factor, Reference Krams, Lees, Hacke, Grieve, Orgogozo and Ford16 (antiinflammatory); fingolimod, natalizumab, minocycline and interleukin-1 receptor antagonist (immune mediated inflammatory response); albumin (augmented blood flow); citicoline (membrane stabilization); fiblast (neuronal healing), and stem cells (neuroregenerative). Reference Rajah and Ding14–Reference Krams, Lees, Hacke, Grieve, Orgogozo and Ford16 This failure to translate benefit to humans is multifactorial, including poor design and methodological weaknesses of many preclinical studies, publication bias, small sample sizes in young healthy animals and models that differ from the typical human condition in mostly elderly patients with comorbidities. Proof of concept (POC) studies, that is, those translating mechanism of action and demonstrating efficacy in man, have, unlike reperfusion studies with lytics and EVT, been notably absent. In clinical trials, many early studies were poorly designed and executed. Neuroprotection was often started too late, and only a minority received thrombolytic or EVT. Reference Babadjouni, Walcott, Liu, Tenser, Amar and Mack17,Reference Chamorro, Dirnagl, Urra and Planas18 The most recent example of failed translation of a promising animal model in rodents and primates is ESCAPE NA-1, in which the eicosapeptide nerinetide, targeting neuronal excitotoxicity, was used in acute stroke patients undergoing EVT. There was no benefit compared to those who received saline, although subgroup analysis showed some benefit in those patients who did not receive concurrent intravenous alteplase. Reference Hill, Goyal and Menon2 It was shown that alteplase resulted in proteolytic cleavage of nerinetide, resulting in a 60% decrease in blood levels compared to those patients who did not receive alteplase. The proposed ESCAPE NEXT trial will adapt the protocol such that patients undergoing EVT will be treated with either placebo or nerinetide but will not receive alteplase as part of their EVT protocol.
The US National Institutes of Neurological Disorders and Stroke established the Stroke Preclinical Assessment Network in 2018 to improve the preclinical development of neuroprotective strategies and facilitate clinical translation. Six study sites and one coordinating center have standardized technical factors, including; central randomization, masking treatment assignment, power analysis and sample size, replication in multiple laboratories, and inclusion of comorbidities. Reference Chamorro, Lo, Renu, van Leyden and Lyden19 The lack of first in man or POC studies worryingly remains a red flag and portends more disappointments despite the increasing list of ongoing trials.
Thrombolysis and EVT were perhaps more translatable because neuroradiology could demonstrate post-treatment recanalization and reperfusion after identifying the target, that is, a large arterial occlusion. The trials were successful because Phase 1 and 2 studies demonstrated not only safely but also the mechanism and efficacy of the intervention, which correlated with improved clinical outcome. With neuroprotective agents like calcium channel blockers and antiinflammatory agents, Phase 2 trials may show infarct reduction but no Phase 1 studies were possible to show translation of the purported mechanism of the agent in humans. Objective demonstration of tissue salvage will be helpful to better design Phase 3 trials.
Ongoing Trials
There are numerous ongoing clinical studies for neuroprotection in acute stroke, including: ESCAPE − NEXT, using nerinetide in patients with acute stroke undergoing EVT, but without alteplase, tenecteplase, or equivalent; MAVARIC, evaluating the use of intraarterial magnesium sulfate and verapamil after successful EVT; RESCUE BRAIN, RESIST, REMOTE-CAT, and REVISE-1 investigating the use of remote ischemic preconditioning, in which ischemia in one organ (the lower limb) leads to ischemic tolerance in another; SONIC, investigating neu2000KWL (salfaprodil), a potent NMDA receptor antagonist and antioxidant, given prior to EVT; RE-HIBER, a Chinese study using regional cerebral hypothermia with chilled saline in association with EVT; DIAGLUICTUS 2, evaluating the utility of peritoneal dialysis after successful EVT to reduce glutamate levels; TESSERACT – BA, looking at transcranial direct current stimulation before and after EVT; TEXAIS and SE-GRACE assessing the value of strict glucose control to limit hyperglycemic damage; OPENS-2, utilizing normobaric hyperoxia in association with EVT; afemelanotide, a potent antioxidant in a POC study in patients with M2 or perforator infarcts who are ineligible for IV alteplase or EVT; CHARM to limit severe cerebral edema with large hemispheric infarction; RNS60, an immune-modulatory agent which has shown some preclinical neuroprotective potential in ALS, and others. Reference Chamorro, Lo, Renu, van Leyden and Lyden19
Therapeutic Hypothermia
TH is pleotrophic and is one of the few interventions that target multiple aspects of acute ischemic brain injury. TH reduces O2 demand (cerebral metabolic rate decreases by 7%−10% for every 1°C cooling), reduces enzymatic degradation, neurotransmitter uptake and intracellular acidosis, and stabilizes membranes. It decreases hyperemia after reperfusion, reduces the production of excitatory amino acids and prevents release of oxidative and nitrosative agents. TH reduces astrocyte and microglial activation, preserves the blood-brain barrier and attenuates the release of pro-apoptotic mediators. Even in later stages, TH can assist postischemic neurogenesis. Reference Karnatovskaia, Wartenberg and Freeman20 Many approaches to achieving TH have been used, including whole body surface cooling, nasopharyngeal sprays, endovascular (inferior vena cava, retrograde internal jugular, and intracarotid) infusions of chilled infusates or ice−cold saline (ICS) and cooled blood, epidural, subdural, and subarachnoid ICS infusion. Reference Wu, Wu, Yang, Xu and Chen21 . Importantly POC studies in humans are achievable.
The physiological risks of systemic TH are legion, and include antiplatelet effects <35°C disturbance of the coagulation cascade <33°C, pneumonia <33°C, electrolyte disorders and cardiac arrhythmias <30°C. Any clinical protocol utilizing TH as a neuroprotectant in acute stroke demands intensive clinical monitoring and physiological support.
Many preclinical studies have shown the benefits of TH in acute stroke models. A systematic review and meta-analysis from 2007 Reference van der Worp, Sena and Donnan22 discussed the results in over 100 publications dating back to 1957, including 3353 animals in focal ischemic models. TH reduced infarct volumes by up to 44%, improved functional outcomes by up to 33% and was most effective with lower temperatures, treatment starting before or at the onset of ischemia and temporary rather than prolonged hypothermia. An updated review from 2016 Reference Dumitrascu, Lamb and Lyden23 discussed another 60 studies, mostly in rodents. Beneficial effects were seen in up to 80% of study animals.
Translation to the bedside however has been problematic. Humans do not tolerate systemic cooling below 32°C without major complications. Whole body cooling by external methods can take hours to induce. Intensive care monitoring for target temperatures <35°C is mandatory. COOLAID in 2004 Reference De Georgia, Krieger and Abou-Chebl24 showed the feasibility of endovascular cooling with delivery of ICS via a catheter in the inferior vena cava. A target temperature of 33°C was reached in 77 min in 13 of 18 patients. The ICTuS-L trial in 2010 Reference Hemmen, Raman, Guluma, Meyer and Gomez25 was the first to investigate endovascular TH with the use of intravenous alteplase. The mean time to achieve target temperature was 67 min in 26 of 28 patients. ReCCLAIM in 2014 used TH to 33C in association with mechanical thrombectomy, achieving target temperature in 64 min. Reference Horn, Sun and Nogueira26 Despite successful clinical translation of the TH intervention none of these studies showed any benefit in the clinical trials. ICTuS II and ReCCLAIM II were both stopped early due to recruitment problems. ReCCLAIM II has re-started a Phase 2 trial in 120 patients using systemic endovascular cooling. Reference Gupta, Jumaa, Badjatia and Yoo27 Recruitment issues also limited the impact of the most ambitious trial of TH in acute stroke, EuroHYP-1. This study aimed to enroll 1500 patients and randomize patients to endovascular cooling to 34−35°C within 6 h of stroke onset and maintained for 12−14 h. Only 98 patients were enrolled over 4.5 years, only 31% achieved the target temperature and 38% had adverse events. There was no difference in the primary outcome (mRS at 90 days) between TH and control groups. Reference van der Worp, McLeod and Bath28 This study demonstrated the difficulties of organizing meaningful trials of TH, including; funding, recruitment, achieving target temperatures, and controlling adverse events like pneumonia and shivering.
A systematic review and meta-analysis from 2020 dating back to 2000 Reference Kuczynski, Marzoughi and Al Sultan29 looked at 12 prospective studies of TH in acute stroke. There were 351 patients in the TH group, achieved by a variety of methods, and 427 controls. The mean time from symptom onset to the start of TH was 6 h. The mean target temperature was 33°C and target body temperature was reached in a mean of 3.5 h. Cooling was maintained for an average of 23 h and 10 studies actively rewarmed patients. Independent functional outcomes did not differ between TH and controls. There were more complications in the TH group, particularly with rapid systemic cooling <2 h. There were trends toward better outcomes in those cooled for longer periods and those who underwent selective rather than systemic TH.
Selective Cerebral TH
Selective TH, whereby the brain alone is cooled has always been a more attractive but technically challenging option. The advantages include a much faster time to reach target temperature and avoidance of systemic complications. The methods to achieve selective TH include ICS infusion, extracorporeal blood cooling, and intracarotid closed-loop cooling. Reference Cattaneo and Meckel30 Animal work with selective TH dates back to the 1950s when Loughheed and Kahn achieved a brain temperature of 20°C in 20 min in dogs, with maintenance of a systemic temperature of 35°C. Reference Lougheed and Kahn31 Contemporary studies using specialized catheters have shown the potential for rapid brain cooling. Reference Mattingly and Lownie32–Reference Mattingly, Denning and Siroen34 A mean brain temperature of 26.5°C was achieved in 30 min in a swine acute stroke model with reduction of MRI stroke volumes compared to controls. Reference Mattingly, Denning and Siroen34 A study in a canine stroke model showed that brain cooling to 31°C could be achieved in less than 25 min, again with a significant reduction in MRI stroke volumes. Reference Caroff, King and Mitchell33 A contemporary study in primates showed a significant linkage between reperfusion status after EVT and efficacy of concurrent selective TH. Reference Wu, Chen, Hussain, Wu and Shi35
Selective TH has been utilized in refractory cardiac arrest Reference Wang, Lin and Chou36 and cerebral aneurysm surgery, Reference Lownie, Menkis, Craen, Mezon, MacDonald and Steinman37,Reference Mattingly, Lopez-Ojeda and Arango38 but set-up times for extracorporeal blood cooling and selective carotid catheterization can take up to 40 min, a disadvantage for use in acute stroke patients. Super-selective TH through microcatheters with infusion of ICS has the advantages of speed and incorporation into standard EVT protocols. Pilot studies have shown safety and feasibility of this approach Reference Chen, Liu and Zhang39,Reference Wu, Zhao and An40 but no clinical efficacy has yet been demonstrated. Some investigators believe that selective TH is the safest and most efficient method to achieve brain cooling, Reference Lyden41 but the logistics of organizing a meaningful trial remain challenging.
The Future
There is growing awareness that the NVU involves more than just neurons, and that any neuroprotective strategy should acknowledge the selective vulnerability of not only neurons but also of astrocytes, oligodendroglia, pericytes, and endothelial cells. Reference Lyden, Lamb, Kothari, Toosi, Boitano and Rajput42 Different cells are affected at different phases to different degrees, and the complex variations in humans are still incompletely understood. The “time window” has been the traditional target leading to optimization of recanalization strategies and interventional devices in clinical trials. This evolved into the concept of “tissue window,” acknowledging that salvageable penumbral tissue can be differentiated from irreversibly damaged brain beyond traditional time boundaries. The differential vulnerability of discrete elements of the NVU has led to the concept of “target window,” a more nuanced approach looking for windows of opportunity with individual cell types at unique time points after the acute ischemic event. Reference Lyden, Buchan and Boltze43 Systemic variables such as age, comorbidities, temperature, glucose levels, circadian effects, and other factors will need to be included in any successful neuroprotection strategy. Reference Chamorro, Lo, Renu, van Leyden and Lyden19 The concept of the NVU is a step toward the ultimate goal of not just neuron or glial protection, but global tissue protection.
Conclusion
If “time is brain,” and EVT approaches the limits of its speed and efficacy, neuroprotection becomes the logical next target and Holy Grail of acute stroke treatment. The mechanisms leading to cell death are multifactorial and it may be that no one neuroprotectant agent is sufficient to impact the ischemic cascade early enough in the process. Perhaps a cocktail of agents addressing specific mechanisms at different time periods may be effective. No matter which neuroprotectant strategy is investigated, it must be integrated with EVT. Reference Savitz, Baron and Yenari44 Drugs that were initially ineffective in clinical trials may be worth repurposing in the context of EVT. Collaboration between acute stroke neurologists and neuro-interventionalists is essential. Reference Neuhaus, Couch, Hadley and Buchan45 As EVT matures and becomes widespread, combining it with neuroprotection becomes the next frontier of acute stroke care.
Acknowledgements
Drs. Stephen P. Lownie and Thomas K Mattingly provided valuable information regarding preclinical and clinical applications of therapeutic hypothermia for neuroprotection in cerebrovascular disorders, including stroke and cerebral aneurysm surgery.
Disclosures
The authors have no conflicts of interest to declare.
Statement of Authorship
AMB: Original stimulus for article, contributor to literature review, editing of manuscript.
DMP: Literature review, primary writing of manuscript.