Background
Epilepsy is a highly prevalent chronic neurological disease. Approximately 50 million people have epilepsy worldwide, with 80% residing in developing countries. 1 Epilepsy is commonly associated with somatic, cognitive and psychiatric comorbidities such as anxiety and depression, which negatively impact quality of life.Reference Rai, Kerr, McManus, Jordanova, Lewis and Brugha 2
Epileptic seizures are unpredictable and sometimes occur without aura, leading to a sense of loss of control with negative effects on self-esteem and often force patients to make significant lifestyle changes. Despite evidence to the contrary, patients and clinicians may suffer the false pretense that physical activity (PA) is injurious to wellbeing.Reference Arida, Scorza, Cavalheiro, Perucca and Moshé 3 Several studies have demonstrated the benefits of PA with respect to improved quality of life, seizure control, mental healthReference Nakken, Løyning, Løyning, Gløersen and Larsson 4 – Reference Arida 6 and a reduction in the interictal epileptiform discharges (IEDs) as seen on the electroencephalogram (EEG).Reference Nakken, Løyning, Løyning, Gløersen and Larsson 4 These findings are encouraging and may support a role for PA as a complementary therapeutic strategy in the treatment of epilepsy.Reference Arida, Scorza, da Silva, Schachter and Cavalheiro 7 , Reference Capovilla, Kaufman, Perucca, Moshé and Arida 8
Material and Methods
We performed a narrative review with the aim of providing a summary of the evidence surrounding risks and benefits of PA in people with epilepsy (PWE). The literature search was performed using Medline®, Embase®, Index Medicus®, Google Scholars, Current Contents and Cochrane databases for articles published from 1960 to July 2017. The search included both medical subject headings and text words for literature on PA and epilepsy. The following keywords were used: activity, activities, athletic, epilepsy, electroencephalogram, EEG, epileptiform discharge, exercise, fitness, game, health behaviors, leisure, leisure time, outdoor, recreational, physical activity, physical effort, physical training, quality of life, recreation, seizure, sports, wellness and wellbeing. We included reviews, original articles and book chapters. Experts were consulted about unpublished studies. Article bibliographies were screened to identify additional sources.
Titles and abstracts were reviewed for original articles regarding effects of PA and sports on epilepsy in animals, children and adults. We included case reports, case series, cohorts, clinical trials and meta-analysis, regardless of language or country of origin. Two authors (JCM, LDL) independently screened and reviewed all the documents.
Five questions were posed:
1) What is the current state of PA in PWE?
2) What are the clinical effects of PA in this population?
3) Does PA reduce the number of IEDs seen on EEG and impact seizure frequency?
4) Which mechanisms related to PA could explain improved seizure control?
5) Can PA adversely affect seizure control?
Results
A total of 167 articles were identified, and after titles and abstracts were reviewed, we excluded 101 documents. Among them, 66 full-text articles were reviewed, including original articles, clinical trials and meta-analysis (see Figure 1).
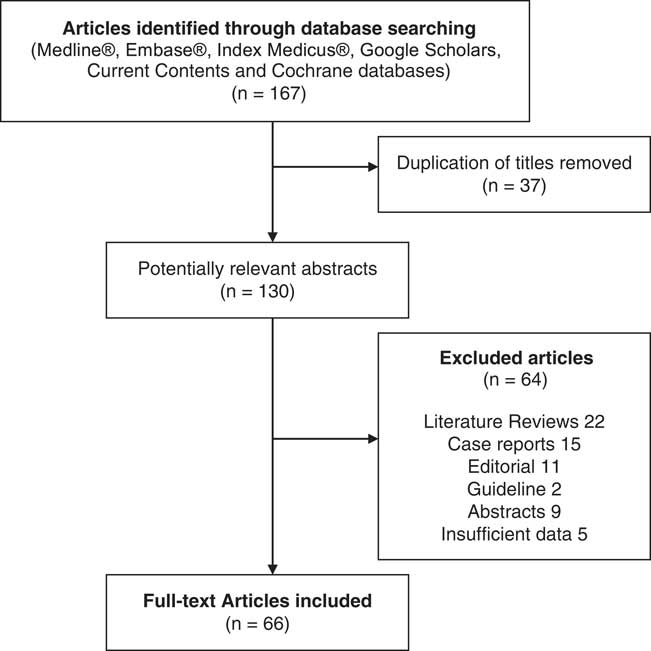
Figure 1 Flowchart of the literature search.
What is the Present State of PA in PWE?
The data on PA and sports in PWE are limited but tend to indicate lower levels of engagement compared with the general population. A survey conducted in Ohio, United States (US) revealed that while 47% of PWE were instructed to be more physically active versus 35% of controls, only 58% of PWE performed PA compared with 76% of controls.Reference Elliott, Moore and Lu 9
Similarly, a Norwegian study reported that sedentary lifestyle was more prevalent in PWE compared with controls (25% vs. 13%, p<0.05).Reference Nakken 10 Another study from the US Midwest documented that PWE performed low-intensity PA ≤3 times per week.Reference Ablah, Haug and Konda 11 A German study demonstrated that controls were more likely to engage in sports on a regular basis (42% of controls vs. 25% of PWE) and less likely to state that they never played sports (15% of controls vs. 31% of PWE). The authors indicated that PWE were instructed to refrain from sports by teachers, instructors and even doctors.Reference Steinhoff, Neusüss, Thegeder and Reimers 12
In their Brazilian study, Arida et al reported that 49% of the PWE did not perform PA on a regular basis. The reasons for this phenomenon included recommendations by relatives, friends and physicians, fear and embarrassment of having a seizure in public, lack of time or motivation, fatigue and absence of company, among others.Reference Arida, Scorza, de Albuquerque, Cysneiros, de Oliveira and Cavalheiro 13 Identical results were seen in a Korean investigation which documented low PA participation owing to factors such as anxiety, polypharmacy and having experienced a seizure during exercise.Reference Han, Choi-Kwon and Lee 14
A Canadian population-based study revealed that 60% of PWE reported being sedentary. Individuals with epilepsy had a higher likelihood of being physically inactive compared with the general population (odds ratio [OR]: 1.4, confidence interval [CI] 95% 1.1-1.7).Reference Hinnell, Williams and Metcalfe 15 The 2010 US National Health Survey revealed that PWE were less likely to follow health guidelines concerning PA. For instance, when asked whether participants had walked at least 10 minutes in the past week, only 39% of PWE indicated they had versus 50% in the general population.Reference Cui, Zack, Kobau and Helmers 16
Wong and Wirrell determined that adolescents with epilepsy were less likely to be involved in sports groups or engaged in PA and were more likely to be overweight and obese than siblings without the disease.Reference Wong and Wirrell 17 In Thailand, 38% of PWE surveyed did not perform PA on a regular basis.Reference Saengsuwan, Boonyaleepan and Tiamkao 18
This trend may be reversible as evidenced by an epilepsy program which enrolled PWE in physical activities in Arizona and was able to achieve a reduction in activity-limited days.Reference Chong, Kudrimoti, Lopez and Labiner 19
Some studies have failed to document differences in activity levels. In Finland, Jalava et al did not detect any significant difference between PWE and controls in terms of PA frequency. Nonetheless, 9% of PWE reported being physically inactive compared with 2% of controls. The same study found performance below expected levels in tests of muscle strength in people with a history of seizures.Reference Jalava and Sillanpää 20 Similar results were reported in another California-based study. However, in this study, PA was not addressed during routine medical consultation in the preceding year in 56% of appointments.Reference Elliott, Lu, Moore, McAuley and Long 21 A Nova Scotia-based Canadian investigation did not relate any difference in PA levels but again revealed that PWE were less likely to participate in sports such as hockey or weight lifting and less likely to engage in PA at home.Reference Gordon, Dooley and Brna 22
The preponderance of data would suggest that PWE are less active than their peers due to a variety of reasons such as prejudice, stigmatization, fear, shame, lack of knowledge or medical advice.
What are the Clinical Effects of PA in This Population?
Animal models confirm a positive effect of PA on mood and seizure control. In a murine model of depression, PA was correlated with a decrease in negative mood symptoms as well as a delay in the development of epilepsy.Reference Epps, Kahn, Holmes, Boss-Williams, Weiss and Weinshenker 23 This finding was associated with the production of galanin, a neuropeptide with antidepressant and anticonvulsive effects.Reference Lerner, Sankar and Mazarati 24
PA and sports participation can have a positive impact on overall health status and quality of life of people living with chronic diseases such as depression,Reference Babyak, Blumenthal and Herman 25 arthritis,Reference Gupta and Aggarwal 26 asthma,Reference Westergren, Fegran, Nilsen, Haraldstad, Kittang and Berntsen 27 hypertension and diabetes.Reference Haxhi, Leto and di Palumbo 28 People with epilepsy tend to be more sedentary than the general population, a finding associated with higher body mass index, minor physical resistance, low self-esteem, and increased likelihood of anxiety and depression. A Brazilian study documented that physical inactivity constitutes a risk factor for the development of depression and anxiety in PWE.Reference de Lima, de Lira and Arida 29
In an Ohio-based study, a 12-week sports intervention program specifically designed for PWE generated enhanced quality of life scores in the PA group (p<0.031) compared with controls (p=0.943). Improvement was seen in variables such as self-image, vitality and emotional state.Reference McAuley, Long and Heise 30 Similarly, a study from the University of Alabama confirmed that adult PWE who practiced regular PA had lower levels of depression.Reference Roth, Goode, Williams and Faught 31 In a Korean study, it was also observed that a program of regular PA in children with benign epilepsy with centrotemporal spikes (BECTS) generated improvements in attention, psychomotor speed, impulse control, inhibition/disinhibition and problem-solving skills.Reference Eom, Lee and Park 5 Overall, exercise seems to exert favorable effects on quality of life, neurocognitive domains and psychosocial functionReference Götze, Kubicki, Munter and Teichmann 32 , Reference Horyd, Gryziak, Niedzielska and Zieliński 33 (Table 1).
Table 1 Studies on physical exercise and epilepsy
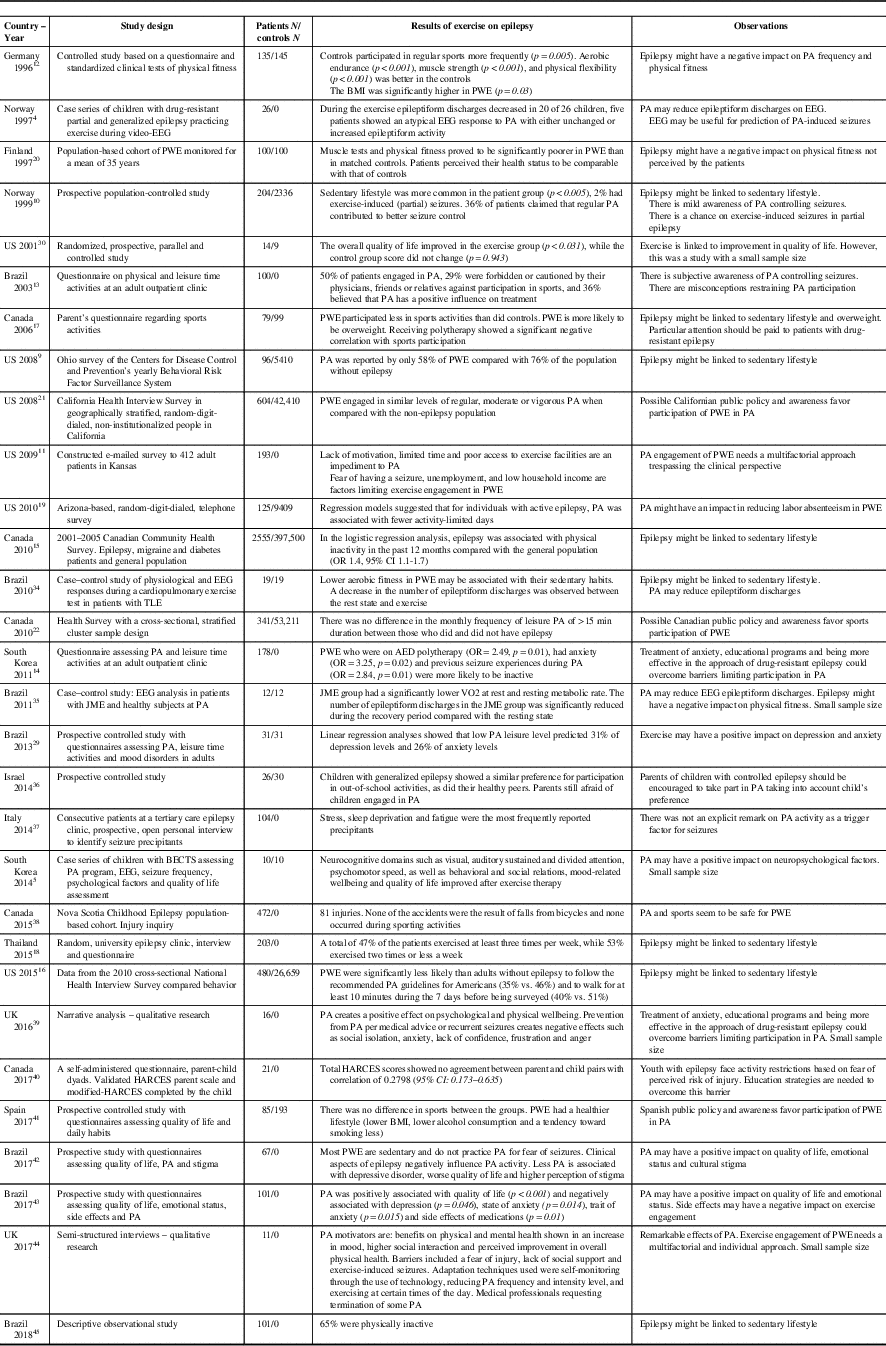
AED=antiepileptic drug; BECTS=benign epilepsy with centro-temporal spikes; BMI=body mass index; HARCES=the Hague Restrictions in Childhood Epilepsy Scale; JME=juvenile myoclonic epilepsy; PA=physical activity; PWE=people with epilepsy.
The pleiotropic effects of antiepileptic drugs (AEDs) must also be considered. Some AEDs such as carbamazepine, lamotrigine, oxcarbazepine, valproate and clobazam can positively impact mood, while others such as levetiracetam, zonisamide, perampanel and phenobarbital can do the opposite. Moreover, some AEDs are known to promote weight gain (i.e., valproic acid, carbamazepine, vigabatrin, gabapentin and phenobarbital), whereas others may be associated with weight loss (i.e., topiramate, zonisamide and felbamate).Reference Ben-Menachem 46 Appropriate AED selection requires joint decision-making between patient and prescriber and a drug’s side-effect profile should be discussed and fully considered before initiation.
Does PA Reduce the Number of IED Seen on EEG and Impact Seizure Frequency?
Electroencephalogram recordings in rats show IEDs decrease or disappearance during PA with returning of the interictal discharges at rest. One hypothesis for this finding is that increased vigilance and attention required in PA may reduce seizure frequency.Reference Hellier and Dudek 47 Basic research has shown that short-duration swimming exerciseReference Tutkun, Ayyildiz and Agar 48 and short-, moderate- and long-duration treadmill exercisesReference Kayacan, Tutkun, Arslan, Ayyildiz and Agar 49 consistently decreased the frequency of penicillin-induced epileptiform activity in male Wistar rats. Likewise, a reduction in the number of clinical and electrographic seizures has been observed with both strengthening (i.e., weightlifting) and aerobic exercises.Reference Radak, Chung and Goto 50 , Reference Peixinho-Pena, Fernandes and de Almeida 51
Findings in animal models may have direct observable clinical implications in humans. For instance, an analysis of video EEG recordings from 26 Norwegian children with epilepsy who attended an exercise program demonstrated a 25% decrease in IEDs in approximately 77% (20/26) of patients during exercise. IEDs increased in relation to baseline following cessation of exercise.Reference Nakken, Løyning, Løyning, Gløersen and Larsson 4 High-intensity exercise has been shown to reduce seizure occurrence and paroxysmal EEG activity in people with temporal lobe and juvenile myoclonic epilepsy.Reference Vancini, de Lira and Scorza 34 , Reference De Lima, Vancini and Arida 35 Moreover, a population-based Swedish study demonstrated that low cardiovascular fitness status at 18 years of age correlated with an increased likelihood of developing epilepsy. The association remained even after controlling for several confounders such as family history, personal history of diabetes, stroke and traumatic head injury. The authors concluded that behaviors that increase cardiovascular fitness may act as positive disease-modifiers against the future development of epilepsy.Reference Nyberg, Aberg, Toren, Nilsson, Ben-Menachem and Kuhn 52
Which Mechanisms Related to PA Could Explain Improved Seizure Control?
Data from animal studies would suggest that the putative neuroprotective effects of PA might be ascribed to various genetic, molecular, biochemical and structural changes.Reference Epps, Kahn, Holmes, Boss-Williams, Weiss and Weinshenker 23 , Reference Hellier and Dudek 47 , Reference Peixinho-Pena, Fernandes and de Almeida 51 , Reference Setkowicz and Mazur 53 , Reference Arida, Scorza, dos Santos, Peres and Cavalheiro 54 The proposed mechanisms include:
1. Release of β-endorphins from the opioid systemReference Contet, Gavériaux-Ruff, Matifas, Caradec, Champy and Kieffer 55
2. Release of steroids secondary to stressReference Arida, Scorza, Toscano-Silva and Cavalheiro 56
3. Increase in melatonin concentrationsReference Mevissen and Ebert 57
4. Increase of parvo albumin in affected cells after seizures. This molecule has been linked to antiepileptogenic effects, cytoprotection and prevention of neuronal death in the affected cells.Reference Arida, Scorza, Scorza, Gomes da Silva, da Graça Naffah-Mazzacoratti and Cavalheiro 58
5. CA1 cells hyperreactivity reduction and generation of structural changes within the hippocampus, which may have an inhibitory effect on the occurrence of abnormal electrical discharges.Reference Arida, Scorza, Scorza, Gomes da Silva, da Graça Naffah-Mazzacoratti and Cavalheiro 58 , Reference Arida, Sanabria, da Silva, Faria, Scorza and Cavalheiro 59
A study using a murine epileptogenic model by Arida et alReference Arida, de Jesus Vieira and Cavalheiro 60 suggested that greater effort was needed to induce epileptogenesis in physical trained animals. The delay in seizure occurrence was attributed to the inhibitory effect of noradrenaline and GABA released during exercise.Reference Westergren, Fegran, Nilsen, Haraldstad, Kittang and Berntsen 27 , Reference Yoneda, Kanmori, Ida and Kuriyama 61 Additionally, exercise resulted in decreased production of oxidants and free radicals.Reference Radak, Chung and Goto 50
In a pentylenetetrazol murine model used to assess the effect of swimming on epileptogenesis after 6 weeks of practice, the exercise group had a greater latency to first seizure, shorter seizure duration, lower amplitude and frequency of IEDs, increased superoxide dismutase activity and non-protein sulfhydryl levels, and greater attenuation of oxidant production.Reference Souza, Oliveira and Furian 62
Similarly, a Polish study that used a pilocarpine murine model of focal epilepsy determined that animals undergoing a PA program had a longer latency in the appearance of status epilepticus and demonstrated lower intensity and shorter seizure duration.Reference Setkowicz and Mazur 53 Possible hypotheses for these observations include increased angiogenesis resulting in decreased excitotoxicity,Reference Black, Isaacs, Anderson, Alcantara and Greenough 63 , Reference Kleim, Cooper and VandenBerg 64 release of neuroprotective trophic factors and expression of neuronal growth factors.Reference Nisticò, Ciriolo, Fiskin, Iannone, de Martino and Rotilio 65 , Reference Carro, Trejo, Busiguina and Torres-Aleman 66
Can PA Adversely Affect Seizure Control?
Sporadic case reports of PA-induced seizures should be weighed against the relatively numerous and well-established benefits of PA (Table 2). Based on scant data, up to 2-10% of PWE may have exercise-induced seizures (defined as seizures occurring in >50% of training sessions).Reference Nakken 10 , Reference Bjørholt, Nakken, Røhme and Hansen 69 These case reports describe patients with both genetic generalized epilepsiesReference Ogunyemi, Gomez and Klass 67 , Reference Werz 73 as well as symptomatic focal epilepsiesReference Eriksen, Ellertsen, Grønningsaeter, Nakken, Løyning and Ursin 71 of frontalReference Simpson and Grossman 68 and temporal lobe origin.Reference Sturm, Fedi, Berkovic and Reutens 72 , Reference Kamel, Badawy and Cook 74
Table 2 Seizure induction by exercise
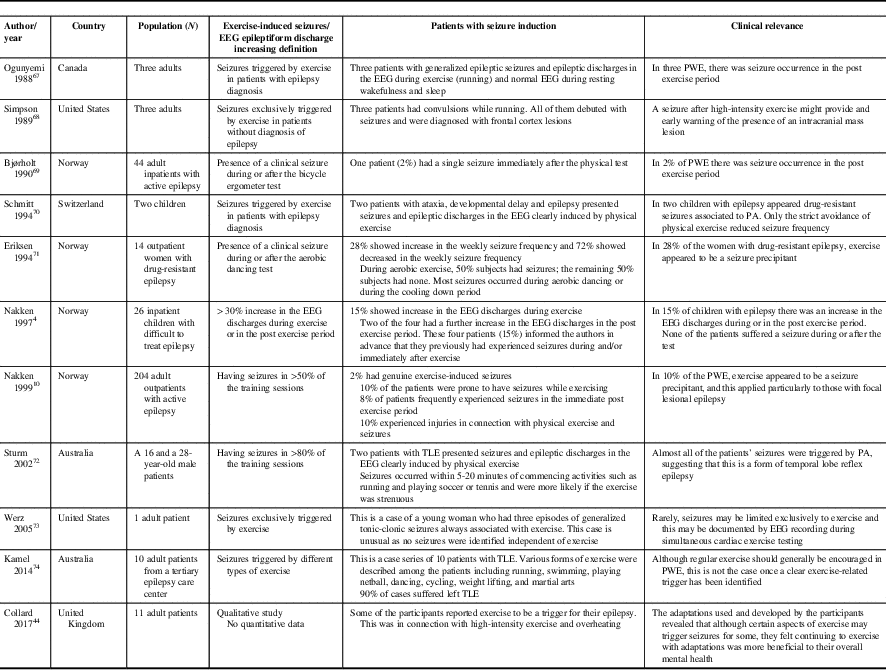
PA=physical activity; PWE=people with epilepsy, TLE=temporal lobe epilepsy.
A review of the limited literature on this subject would indicate that the occurrence of exercise-induced seizures is overall quite rare.Reference Nakken, Løyning, Løyning, Gløersen and Larsson 4 , Reference Nakken 10 Of interest, PA-induced seizures have been mainly attributed to high-intensity exercise such as ball games, jogging, running and hiking. In addition, high altitude may also provoke seizures, due in part to hypoxia and hypocapnic hyperventilation.
Some patients are reported to present with a high frequency of seizures happening either often (>80% of the time) or exclusively occurring during PA. The authors surmise that these patients may have a form of temporal lobe reflex epilepsyReference Schmitt, Thun-Hohenstein, Vontobel and Boltshauser 70 , Reference Sturm, Fedi, Berkovic and Reutens 72 , Reference Kamel, Badawy and Cook 74 and that the temporal lobe might be uniquely more sensitive to the generation of exercise-induced IEDs compared with other cortical areas. In these cases, complex partial seizures might be most susceptible to activation during exercise.Reference Bennett 75
Arida et alReference Arida, Cavalheiro, da Silva and Scorza 76 correlated the occurrence of PA-induced seizures with homeostatic alterations linked to general fatigue, psychic stress of competition, hypoxia, hypoglycemia, hyperhydration, hyponatremia and hyperthermia. It is known, for instance, that hyperventilation may frequently induce absence seizures and some focal seizures in patients at rest. It has been theorized that resting hyperventilation might trigger seizures due to respiratory alkalosis. However, this explanation is unlikely to be compelling in the case of exercise, which tends to result in metabolic acidosis.
Discussion
Epilepsy is associated with reduced sports participation and PA in PWE. Family, peers and educators may discourage PA due to the mistaken belief that epileptic seizures during exercise could lead to psychological and social stigma and adversely impact quality of life.Reference Rodenburg, Meijer and Scherphof 77 , Reference Mecarelli, Messina and Capovilla 78 As PWE replace “outdoor” activities with “indoor” sedentary programs, physical exercise levels may further decline.Reference Painter, Rausch and Modi 79
Healthcare teams should extol the virtues of PA, which include: (1) better social integration, (2) improvement of depression and anxiety, (3) protection against osteopenia/osteoporosis, (4) enhanced sleep, (5) positive effects on quality of life and (6) possible reduction in seizure frequency.Reference Arida, Cavalheiro, da Silva and Scorza 76
Education on the topic is essential and should extend to community, educators, patients and families. To achieve greater participation of PWE in sports, necessary infrastructure must be developed addressing issues such as accessibility, transportation and safety.Reference Capovilla, Kaufman, Perucca, Moshé and Arida 8
The International League Against Epilepsy (ILAE) has proposed some recommendations concerning PA in children: in aquatic sports, one should weigh risks and benefits; in high-altitude sports such as rock climbing or tree climbing, it is noted that “regardless of whether the child has epilepsy, common sense prevails”; cycling, skating or skateboarding should be limited if there is inadequate seizure control or if epilepsy was recently diagnosed; on contact sports the ILAE states that mild traumatic head injury is unlikely to precipitate an epileptic seizure; finally, diving, parachuting and similar sports should be avoided. 80
In 2016, the ILAE published a report that divided sports into low, moderate and high-risk categories. The level of risk should be considered along with variables such as type of sport, probability of seizures, type and severity of seizures, precipitating factors, diurnal variation, protective measures and patient risk tolerance. Ultimately, the report eschews blanket recommendations in favor of a patient-specific approach.Reference Capovilla, Kaufman, Perucca, Moshé and Arida 8 It is advised that high-performance athletes with epilepsy should inform coaches, training staff and sport committees about their pharmacotherapy to protect themselves from charges of doping.Reference Kaufman 81
Rarely, exercise can have an activity on seizure occurrence,Reference Ogunyemi, Gomez and Klass 67 – Reference Kamel, Badawy and Cook 74 and clinicians must be attuned to this possibility. In some cases, the possibility of a temporal reflex epilepsy must be considered, and clinicians should ask their patients whether specific activities lead to seizures. If a specific PA is identified as a trigger, it is advisable to record an EEG during this activity. Should such an activity be associated with the occurrence or increase in IEDs or result in seizure, avoidance may be prudent. Alternatively, the patient and clinician may also consider reducing frequency and intensity of the specific exercise, greater supervision during the activity and initiation of preventive antiepileptic therapy.
Conclusions
People with epilepsy are less physically active and less likely to be involved in sports than their peers. Data supporting the beneficial effects of PA on seizure control are insufficient. However, various positive effects on neuropsychological, emotional and quality of life measures have been documented. There is intriguing data to suggest that PA may reduce IED frequency and seizure occurrence, and thereby serve as a useful adjunctive therapy for the treatment of epilepsy. Stakeholders must be aware of various clinical, psychological and socio-economic barriers that limit PA in PWE. The benefits of PA should be analyzed as part of a holistic approach to patient health.
Statement of Authorship
Study concept and design: JCM, LDL. Literature review: DMCV, JPOH, VBC, LDL. Interpretation of data: JFTZ, JCM, LDL, SR. Drafting of manuscript and revisions: JFTZ, JCM, LDL, SR, VBC.
Disclosures
JFTZ receives grants from the University of Saskatchewan, the Royal University Hospital Foundation in Saskatoon, Saskatchewan, and the Saskatchewan Health Research Foundation. JCM, LDL, SR, VBC, DMCV and JPOH have nothing to disclose. None of the authors have any conflict of interest to declare.





