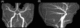A specific mutation (Arg179) of the ACTA2 gene has previously been described to cause a syndrome of multisystemic smooth muscle dysfunction with an extremely characteristic cerebrovascular appearance.Reference Munot, Saunders and Milewicz1 Accurate neuroimaging diagnosis of this entity is important as this syndrome predisposes to complications such as early-onset ischemic stroke and ascending thoracic aortic aneurysm.Reference Guo, Papke and Tran-Fadulu2, Reference Milewicz, Ostergaard and Ala-Kokko3 The following case demonstrates a previously undescribed ACTA2 mutation (Met46) with an identical cerebrovascular imaging appearance to that of Arg179 mutations, but a less severe overall phenotype.
A 19-year-old male with history of patent ductus arteriosus requiring ligation presented for a routine health maintenance exam, where abdominal and femoral bruits were heard. An echocardiogram then demonstrated a mildly dilated aortic root measuring 3.7–4.0 cm, and MRA of the body demonstrated left common iliac artery stenosis. MRA of the head and neck demonstrated dilation of the petrous and cavernous portions of the internal carotid arteries bilaterally, with abrupt tapering of the clinoid and supraclinoid segments, and an abnormally straightened appearance of the posterior, middle, and anterior cerebral arteries (Figure 1). A follow-up MRI performed a year later demonstrated a few scattered foci of T2 prolongation in the white matter, an imaging feature often seen in patients with ACTA2 gene mutations from small vessel ischemic injuries (Figure 2).

Figure 1: Coronal (A) and sagittal (B) maximum intensity projection imaging from head-and-neck MRA demonstrates dilation of the extradural carotid arteries bilaterally, with abrupt tapering of the intraclinoid carotid artery, and straightened posterior, middle, and anterior cerebral arteries.
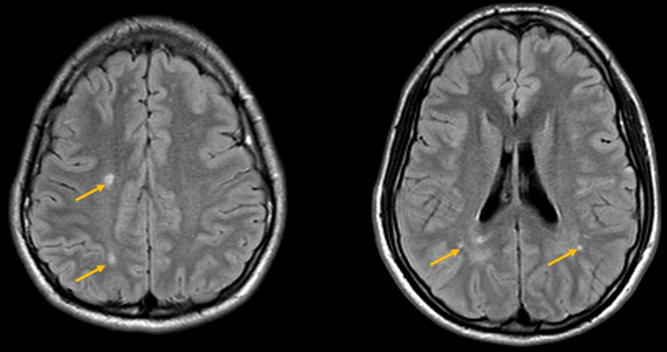
Figure 2: Follow-up MRI brain, performed a year later, demonstrates a few scattered foci of T2 prolongation in the white matter (yellow arrows).
An aortopathy gene panel test was performed to evaluate the following genes: ACTA2, CBS, COL3A1, COL5A1, COL5A2, FBN1, FBN2, FLNA, MAT2A, MEDI2, MFAP5, MYH1, MYLK, NOTCH1, PRKG1, SK1, SLC2A10, SMAD3, SMAD4, TGFB2, TGFB3, TGFBR1, and TGFBR2. Results confirmed a heterozygous Met46Arg mutation in the ACTA2 gene, which at the time was characterized as a “variant of uncertain significance.” Of note, this genetic variant has now been classified as a likely pathogenic variant based on review of data in context of the 2015 ACMG Standards and Guidelines for the Interpretation of Sequences Variants.Reference Richards, Aziz and Bale4 Further clinical evaluation found no additional signs or symptoms of multisystemic smooth muscle dysfunction syndrome, such as congenital mydriasis, hypotonic bladder, or pulmonary hypertension, as has been described previously with other ACTA2 mutations.Reference Munot, Saunders and Milewicz1–Reference Milewicz, Ostergaard and Ala-Kokko3 The parents tested negative for the Met46Arg mutation in the ACTA2 gene, indicating that this mutation likely arose de novo.
ACTA2 mutations can have characteristic cerebrovascular findings, allowing the neuroradiologist to suggest a specific genetic diagnosis based on imaging findings alone. These cerebrovascular findings have previously been specifically attributed to heterozygous Arg179 ACTA2 mutations, and include ectasia and stenosis of the internal carotid artery, a straightened course of the cerebral arteries, and an absence of basal collateral vessels.Reference Munot, Saunders and Milewicz1 However, unlike our case, Arg179 ACTA2 mutations cause a more diffuse syndrome of multisystemic smooth muscle dysfunction.Reference Milewicz, Ostergaard and Ala-Kokko3 This novel case demonstrates a previously undescribed ACTA2 mutation with cerebrovascular features identical to Arg179 ACTA2 mutations, but a less severe overall phenotype.
Disclosures
The authors have no conflicts of interest to declare.
Statement of Authorship
All authors contributed equally to the manuscript.