CLINICIAN'S CAPSULE
What is known about the topic?
Among children, anaphylaxis is an increasingly common, life-threatening allergic reaction and there are deficiencies in both diagnosis and management.
What did this study ask?
Canadian pediatric emergency medicine practitioners were surveyed to assess the knowledge and management gaps for anaphylaxis, as per evidence-based, best practice guidelines.
What did this study find?
One-half of the physicians surveyed recognized all three anaphylaxis cases and administered epinephrine, where indicated, each time.
Why does this study matter to clinicians?
Recognition of anaphylaxis without urticaria or pulmonary findings, evidence-based management, and repeat epinephrine for ongoing anaphylaxis needs to be improved.
INTRODUCTION
Anaphylaxis is a serious systemic hypersensitivity reaction that is usually rapid in onset and may cause death.Reference Simons, Ardusso and Bilò1,Reference Turner, Worm and Ansotegui2 The highest incidence of anaphylaxis is in children and adolescents.3,Reference Simons and Sampson4 In Canada, there is an emergency department (ED) visit for food allergy approximately every 10 minutes,5 and up to 80% of anaphylactic reactions in children are triggered by food.Reference Gabrielli, Clarke and Morris6 Furthermore, 8% of allergy-related ED visits are due to anaphylactic shock.3
Despite the frequency and seriousness of anaphylaxis, international studies continue to report practice gaps in management with both difficulty in recognizing anaphylaxis as well as deviation from evidence-based, best practice guidelines.Reference Pouessel, Galand and Beaudouin7–Reference Kastner, Harada and Waserman10 The literature indicates that even when anaphylaxis is recognized, there is undertreatment with epinephrine, overtreatment with adjunctive medications, and inadequate patient education and follow-up.Reference Lee, Enarson and Clarke11–Reference Cheng, Farrell and Friedman16
The magnitude of these gaps among Canadian pediatric emergency medicine (PEM) physicians is unknown. We proposed to explore the national practice pattern in the management of children with anaphylaxis. The primary objective of this study was to examine the self-reported practice of pediatric emergency physicians across Canada with regard to the diagnosis of anaphylaxis and use of epinephrine. Secondary objectives were to explore the pediatric ED anaphylaxis resources, such as established treatment pathways (e.g., order sets) and patient education materials.
METHODS
Study design
We conducted a national census of physicians in the Pediatric Emergency Research Canada (PERC) physician database. PERC, a cross-Canada network of pediatric EDs, was formed to conduct multicentre research designed to improve the health of childrenReference Bialy, Plint and Zemek17 and maintains a database of physicians who work within member institutions. The Research Ethics Board of the Children's Hospital of Eastern Ontario Research Institute approved this study.
Study setting and population
All physicians listed in the PERC physician database were surveyed; this includes both the attending physicians and fellows in training. PERC includes 13 sites across Canada. The PERC physician database contains about 70% of the physicians currently working in pediatric EDs. Two hundred fourteen members were surveyed. The annual census for EDs participating in PERC ranges from 17,000–82,000 visits per year.
Survey instrument
The vignette-based, multiple choice survey tool was developed de novo. We first undertook a literature review to identify the recurring themes of gaps in anaphylaxis diagnosis and management.Reference Kastner, Harada and Waserman10,Reference Sicherer, Allen and Lack15 Based on this literature review, we then developed clinical vignettes, adapted from clinical experience and case reports, that mapped to the three separate National Institute of Allergy and Infectious Diseases (NIAID) criteria (supplemental material Appendix 1). Three vignettes matched the three NIAID anaphylaxis criteria: acute onset of skin or mucosal tissue symptoms associated with respiratory changes, acute onset of skin and gastrointestinal manifestations after exposure to a likely allergen, and anaphylactic shock with hypotension after exposure to a known allergen.Reference Simons, Ardusso and Bilò1,Reference Simons, Ebisawa and Sanchez-borges18,Reference Simons, Ardusso and Dimov19 A fourth vignette, a case of food protein-induced enterocolitis syndrome (FPIES) but not anaphylaxis, was added to further assess the diagnostic ability of survey participants. The multiple-choice questions associated with diagnosis reflected confidence in identifying anaphylaxis (modified Likert scale), use of epinephrine, use of adjunctive medications, outpatient prescribing, and follow-up. There was a text option for participants to add non-listed answers. Additional multiple-choice questions reflected epinephrine dosing, observation time, and respondent demographics. The 13 PERC site representatives received an additional five multiple-choice questions addressing ED volumes and availability of anaphylaxis resources in the department, such as treatment pathways and patient education materials.
The following measures were done to ensure the quality of the survey instrument:Reference Burns, Duffett and Kho20 pretesting with three PEM physicians within the author's department, review of survey instrument by a statistician and allergy specialist, and, finally, pilot testing with four PEM physicians and three trainees from three centres across Canada. Changes were made to list epinephrine concentrations in multiple units, include additional adjunctive medications, and specify time intervals between exposure and reactions. The survey was translated into French and retested with a Francophone. The final step was review and approval by the PERC executive.
Sampling procedure
A self-administered, combined-mode survey (web-based and mail send-out for non-responders) was used (supplemental material Appendix 2). Participation was exclusively voluntary, with explicit consent to be approached for surveys inherent in the PERC physician database membership. The electronic questionnaires were distributed through REDcap®, a secure web-based application that is used for building surveys and managing online databases. The survey send-outs were implemented based on modified Dillman's Tailored Design Method survey, with a target response rate of greater than or equal to 70%. This method started with a pre-survey electronic announcement, which was sent in the spring of 2018, and then two Internet mailings at specified two-week intervals, with reminders after each. A unique link was sent to each participant to enable tracking. A third and final paper-based survey was mailed to individuals who did not respond to the electronic survey. The survey timeframe was from May to July 2018. All participants were offered a $5 coffee gift card incentive to complete the survey, which could be converted to a donation to PERC or declined if so desired. Survey responses were aggregated in order to protect the identity of survey respondents.
Outcome measures
Our primary outcomes of interest were the correct diagnosis of anaphylaxis and treatment of anaphylaxis with epinephrine, where indicated. Secondary outcomes of interest were epinephrine prescribing practices, adjunctive medication use, practice variation in observation time and admission to hospital, and the availability of resources in the ED, such as treatment pathways and patient education materials.
Sample size and analysis
The questionnaire was administered to all PERC members (n = 214). Descriptive statistics were produced for all variables. Categorical variables were summarized using frequencies and percentages. Vignette-specific response choices of “definitely yes” and “probably yes” were grouped as “yes,” and “definitely no” and “probably no” were grouped as “no.” Proportions, with 95% confidence intervals (CI), and aggregate variables were calculated. We performed a univariate analysis using Pearson's chi-square test to explore variables associated with the primary outcome (practice setting, training designation, or years in practice). The significance level was determined as p < 0.05. All statistical analyses were performed using R statistical software version 3.5.2 (R Core Team, Vienna, Austria).21
RESULTS
Of the 214 members invited to participate in the survey, 152 (71%) responded. The demographic characteristics of those participants are summarized in Table 1. Most respondents work in pediatric EDs (96%) and were trained through PEM Fellowships (63.5%), followed by pediatrics (17%) and emergency medicine (11%). The majority of respondents were from Ontario Quebec and Alberta, respectively (see Table 1). There was an even distribution of respondents across career experience in five-year intervals.
Table 1. Demographic characteristics of study participants*
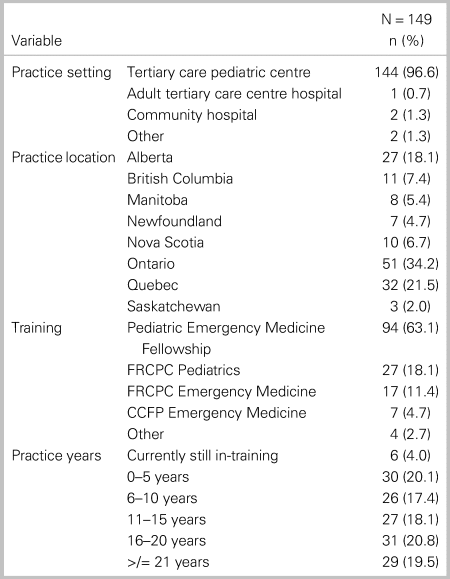
Note: *3 of 152 respondents with missing values.
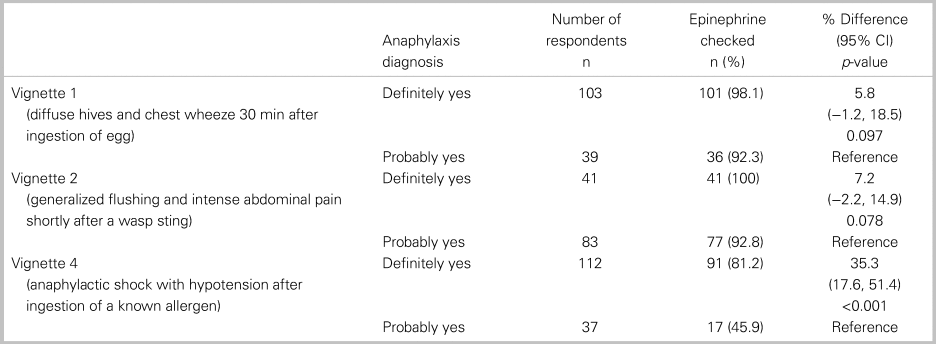
Of all 152 respondents, 115 or 76% (95% CI: 69, 82) accurately diagnosed all three cases of anaphylaxis, and 82 or 54% (95% CI: 46, 62) treated with epinephrine each time it was indicated. In a comparative analysis, there was no significant relationship between the primary outcome and any of the following factors: practice setting, training designation, or years in practice (Table 2). Table 3 shows participant responses to the diagnosis and management of all clinical vignettes. Respondents were able to accurately recognize anaphylaxis 93% (95% CI: 88, 96), 82% (95% CI: 75, 87), and 99% (95% CI: 96, 100) of the time for the NIAID criteria one through three, respectively (see Table 3). However, of those who correctly identified the anaphylaxis vignettes, epinephrine treatment was appropriately prescribed 96% (95% CI: 92, 98), 95% (95% CI: 90, 98), and 72% (95% CI: 65, 79) of the time for NIAID criteria one through three, respectively. It is important to note that, in vignette 3, the patient received an auto-injector dose of epinephrine prior to ambulance transport; however, the patient was persistently in hypotensive anaphylactic shock by arrival at the hospital, therefore repeat epinephrine administration was indicated in this case. Except for vignette 4, there was no difference between participants’ degree of confidence (i.e., definitely versus probably) with the diagnosis of anaphylaxis and epinephrine administration (Table 4).
Table 2. Association between participants’ characteristics with correct diagnosis and treatment of anaphylaxis vignettes
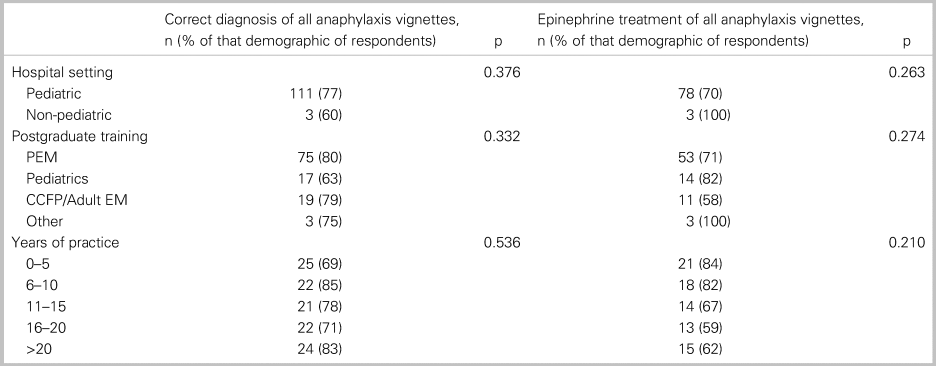
Table 3. Summary of clinical vignettes diagnoses and treatments (n = 152 respondents)
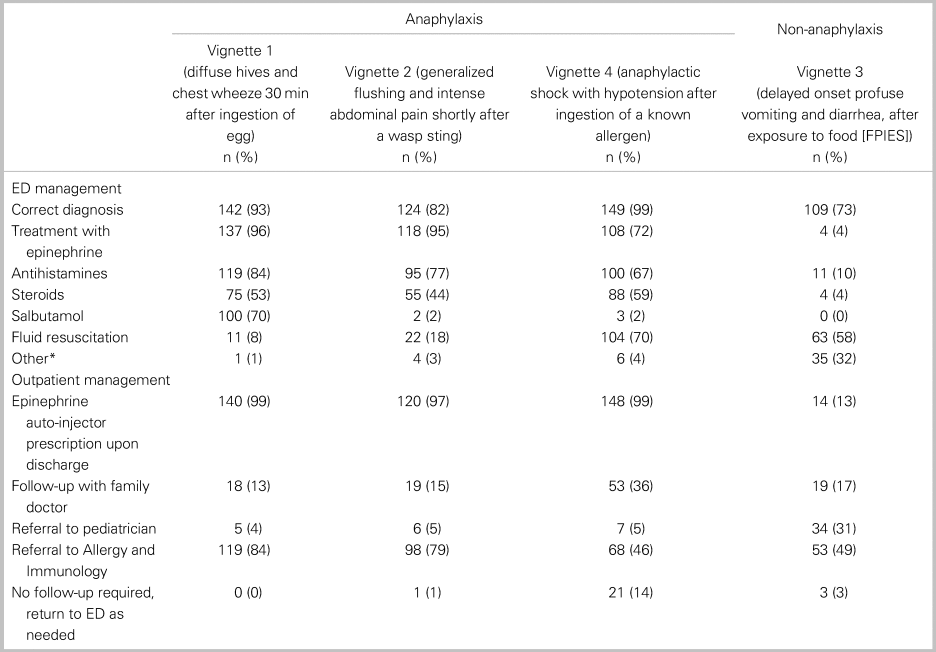
Note: *all respondents indicated ondansetron.
Table 4. Comparing the proportion of epinephrine prescribing between “definitely yes” and “probably yes”
Adjunctive treatments with antihistamines and corticosteroids were prescribed with the following frequencies in patients diagnosed with anaphylaxis: antihistamines 84% (95% CI: 77, 89), 77% (95% CI: 68, 83), and 67% (95% CI: 59, 74) for criteria one through three, respectively, and corticosteroids in 53% (95% CI: 45, 61), 44% (95% CI: 36, 53), and 59% (95% CI: 51, 67) for criteria one through three, respectively. The most frequently prescribed formulations of antihistamine and corticosteroids were diphenhydramine and dexamethasone, respectively. Among the appropriately diagnosed anaphylaxis cases, over 96% of participants provided discharge prescriptions for epinephrine. Outpatient referral to allergy and immunology varied between 80% for vignette 1 and 46% for vignette 3 (see Table 3).
Table 5 outlines the epinephrine dosing selected, length of observation, and department resources reported by respondents. The correct formulation of intramuscular (IM) epinephrine in treating anaphylaxis was identified by 86% (95% CI: 80, 91) of the participants. The duration of ED monitoring after epinephrine administration varied based on whether the patient received one or two doses of epinephrine. After one epinephrine administration, 77% of survey participants observed for 4–8 hours. After two doses of epinephrine, practice variation was wide: 53% observed for 4–8 hours, 20% for >8 hours, and 24% would admit the patient to hospital.
Table 5. Epinephrine dose and management (n = 152 respondents)
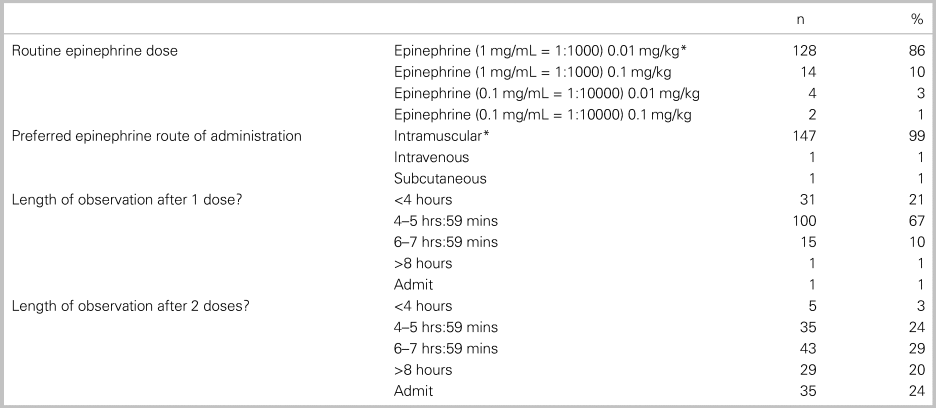
Note: *Correct answer.
With regard to the availability of anaphylaxis order sets and patient education resources, 4 of the 11 (36%) PERC site representatives reported that their centres have established anaphylaxis order sets, and only 1 site indicated having patient discharge communication resources, such as anaphylaxis emergency action plans and epinephrine auto-injector discharge prescription sets.
DISCUSSION
Interpretation of findings
Although the majority of PEM physicians have appropriate knowledge for the diagnosis and management of pediatric anaphylaxis, a substantial portion have knowledge gaps that may adversely impact the care of this vulnerable population. In particular, the recognition of anaphylaxis without urticaria or pulmonary findings and administering repeat doses of epinephrine for ongoing cardiorespiratory compromise are significant gaps that we identified. Across Canadian pediatric EDs, we identified a lack of structured treatment pathways, such as order sets and a lack of structured patient education or discharge resources.
Previous studies
The proportion of PEM physicians who accurately diagnosed all cases of anaphylaxis in our survey is similar to previous studies with self-reported practice patterns. For example, 70% of general practitioners in FranceReference Pouessel, Galand and Beaudouin7 and 70% of general pediatricians in the United StatesReference Krugman, Chiaramonte and Matsui8 demonstrated successful recognition of cases of anaphylaxis. Similarly, in our previous survey of over 300 Canadian physicians who practise in adult or general EDs, 62% correctly identified pediatric anaphylaxis scenarios.Reference Alqurashi9 Although ED studies found the overall performance of the anaphylaxis diagnostic criteria to be reasonably accurate, the interpretation and application of the individual criterion in clinical practice, particularly in the pediatric population, remain challenging and likely contribute to misdiagnosis.Reference Brauer, Motosue and Li22 A study by Wang et al. found that, while 85% of physicians correctly identified the case of anaphylaxis with prominent skin and respiratory symptoms, only 61% recognized the case without skin and respiratory symptoms as being anaphylaxis.Reference Wang, Young and Nowak-Wegrzyn23 Our study also underscores this challenge, with a 93% correct diagnosis of vignette 1 (urticaria and respiratory symptoms) compared with an 82% correct diagnosis of vignette 2 (flushing and gastrointestinal symptoms after a wasp sting).
Epinephrine is the most important treatment for anaphylaxis. Several studies have shown the critical role of prompt administration of IM epinephrine in the prevention of severe and fatal anaphylaxis.Reference Bock, Munoz-Furlong and Sampson24–Reference Ko, Kim and Seo27 Interestingly, however, our survey identifies a discrepancy between diagnosis and treatment of anaphylaxis. While the treatment with epinephrine for the individual anaphylaxis vignettes is higher than previous studies,Reference Lee, Enarson and Clarke11,Reference Hochstadter, Clarke and De Schryver12,Reference Hemler and Sharma14 only 53% of the physicians who correctly identified all three cases of anaphylaxis treated with epinephrine each time that it was indicated. This discrepancy towards undertreatment was more marked in the case of hypotensive anaphylactic shock that required repeat epinephrine dosing for ongoing hypotension. Although nearly all participants made the correct diagnosis, only 72% treated with epinephrine. Whether this is related to a lack of knowledge, apprehension about administering repeated doses of IM epinephrine, or a failure to recognize hypotension for age is unclear.
Strengths and limitations
This is the first national survey that has explored anaphylaxis practice patterns of Canadian PEM physicians and we had a high response rate. The inherent limitation in this study, as with any survey of practice pattern, is self-reporting. Although self-reported anaphylaxis management might not accurately reflect actual practice, but rather what is perceived as the expected practice, our findings are consistent with the data from a Canadian anaphylaxis registry, which showed that 48.2% of children presenting with anaphylaxis to the ED were treated with epinephrine.Reference Gabrielli, Clarke and Morris6 The identified practice gaps are concerning even if we assume that the reported practice represents the “best,” rather than the “real” practice of PEM physicians. This study is also limited by potential coverage error as most, but not all, practicing physicians in PEM were approached. Finally, since we purposefully did not ask individual participants in which Canadian pediatric centre they practise to avoid identifying individual respondents, we are not able to explore the association between the presence of order sets and the primary outcome.
Clinical implications
This study highlights specific gaps in practice, particularly the increased challenge of diagnosing anaphylaxis without urticaria or pulmonary findings. To address this critical issue, in 2019, the World Allergy Organization (WAO) recently refined the diagnostic criteria for anaphylaxis. The updated criteria reflect the reality that the occurrence of hypotension or objective respiratory signs, in isolation, following exposure to a known or highly probable allergen is indicative of anaphylaxis.Reference Turner, Worm and Ansotegui2 Translating Emergency Knowledge for Kids (TREKK) is a national centre for knowledge translation.28 The TREKK recommendations for anaphylaxis are consistent with the WAO update.Reference Campbell29 Therefore, clinicians are encouraged to adopt these updated criteria in clinical practice to overcome the diagnostic dilemma and ultimately improve the management of anaphylaxis.
Several recent studies investigated the utility of corticosteroids as adjunctive therapy for anaphylaxis.Reference Alqurashi and Ellis30 Corticosteroids have not been shown to reduce the severity of initial anaphylaxis or reduce the risk of biphasic reactions.Reference Shaker, Wallace and Golden31 In fact, recent evidence showed that prehospital use of corticosteroids is associated with admission to hospital and the intensive care unit, even after adjusting for severity, treatment with epinephrine and antihistamines, asthma, sex, and age.Reference Gabrielli, Clarke and Morris6 Further, a meta-analysis by Shaker et al. found that compared with adults, children who received corticosteroid therapy for the initial reaction were at higher risk of developing a biphasic reaction (odds ratio [OR], 1.5; 95% CI: 1.01, 2.38).Reference Church, Maurer and Simons32 Therefore, corticosteroids should not be routinely administered to children with anaphylaxis.Reference Campbell29 Similarly, due to the safety concerns of first-generation H1 antihistamines, such as diphenhydramine, these agents should not be used in the treatment of anaphylaxis. These concerns are also outlined in the TREKK recommendationsReference Campbell29 and the position statements from several scientific societies.Reference Fein, Fischer, O’Keefe and Sussman33,Reference Rotter, Kinsman and James34 These guidelines recommend newer generation antihistamines as a safe and effective alternative to first-generation H1 antihistamines.
Clinical pathway and preprinted orders have emerged as a potentially important knowledge dissemination strategy to promote patient safety and evidence-based healthcare practice.Reference Kozer, Scolnik, MacPherson, Rauchwerger and Koren35–Reference Simons, Ardusso and Bilò37 The free, open-access anaphylaxis resources developed by TREKK target several aspects of the practice gaps identified in our survey.
Research implications
The wide variation in practice for the duration of ED monitoring confirms a high degree of uncertainty regarding the best and most cost-effective disposition practices. This clinical uncertainty originates from the lack of validated clinical predictors for biphasic anaphylaxis. The international research agenda for anaphylaxis recognized this critical gap in current knowledge and called for prospective studies to derive a robust risk stratification model.Reference Church, Maurer and Simons32
CONCLUSIONS
The majority of respondents recognized cases of anaphylaxis; however, there were demonstrated gaps in management that may adversely impact this vulnerable population. The recognition of anaphylaxis without urticaria or pulmonary findings and administering repeated doses of epinephrine for ongoing cardiopulmonary compromise are the most significant identified gaps. In order to improve the uptake of evidence-based best practice guidelines, there is an urgent need for knowledge dissemination and the uptake of departmental resources to facilitate this implementation.
Acknowledgements
Pediatric Emergency Research Canada (PERC) and the CHEO Research Institute contributed to this work.
Supplemental material
To view supplementary material for this article, please visit https://doi.org/10.1017/cem.2020.458.
Financial support
This study was supported by a research grant from the CHEO Research Institute.
Competing interests
None declared.









